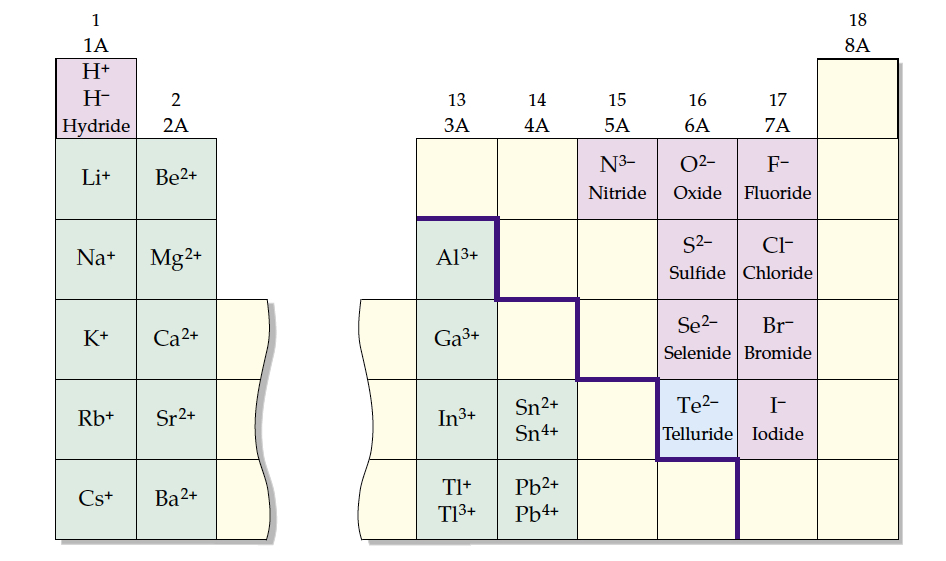
Magnesium Telluride Ionic Formula. The table shows element percentages for mgte (magnesium telluride). The chart below shows the calculated isotope pattern for the. You then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down its. Copyright ©2024 infospace holdings llc, a system1 company. The chemical formula for magnesium telluride is mgte. Data at other public nist sites: Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Magnesium and nitrogen react to form an ionic compound. Although both of these ions have higher charges than the ions.
from myweb.liu.edu
Predict which forms an anion, which forms a cation, and the charges of each ion. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Magnesium and nitrogen react to form an ionic compound. Copyright ©2024 infospace holdings llc, a system1 company. You then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. The chemical formula for magnesium telluride is mgte. The table shows element percentages for mgte (magnesium telluride). Although both of these ions have higher charges than the ions. Then, identify the anion and write down its. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
Chapter 2 Atoms, Molecules and Ions
Magnesium Telluride Ionic Formula You then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down its. The chemical formula for magnesium telluride is mgte. Data at other public nist sites: The chart below shows the calculated isotope pattern for the. Although both of these ions have higher charges than the ions. You then figure the formula based on the lowest whole number ratio of cations to anions that produces a neutral formula. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The table shows element percentages for mgte (magnesium telluride). Copyright ©2024 infospace holdings llc, a system1 company. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion.
