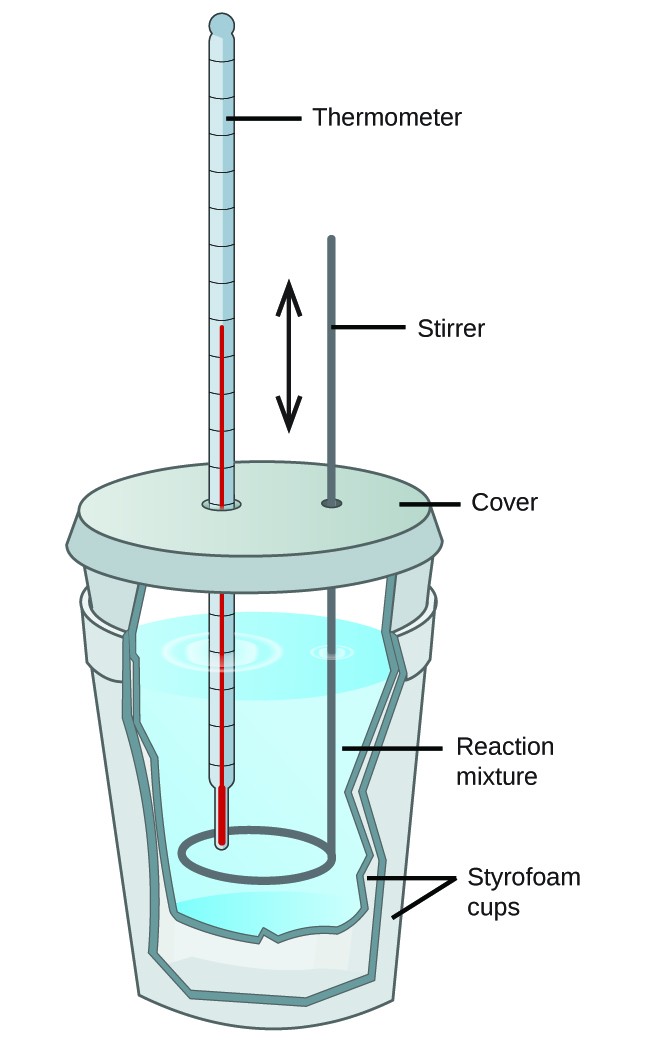
Chemistry Definition Of Calorimeter . For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. By knowing the change in heat, it can be. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from courses.lumenlearning.com
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. By knowing the change in heat, it can be.
Calorimetry Chemistry Atoms First
Chemistry Definition Of Calorimeter For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in heat, it can be. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.thoughtco.com
Calorimeter Definition in Chemistry Chemistry Definition Of Calorimeter By knowing the change in heat, it can be. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount. Chemistry Definition Of Calorimeter.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in heat, it can be. In chemistry and thermodynamics, calorimetry (from latin calor. Chemistry Definition Of Calorimeter.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Chemistry Definition Of Calorimeter By knowing the change in heat, it can be. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical. Chemistry Definition Of Calorimeter.
From www.pinterest.com
Calorimeter Chemistry classroom, Chemistry lessons, Physics classroom Chemistry Definition Of Calorimeter For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical,. Chemistry Definition Of Calorimeter.
From exollkmhz.blob.core.windows.net
Define Adiabatic Calorimeter at Alma Martin blog Chemistry Definition Of Calorimeter For example, when an exothermic. By knowing the change in heat, it can be. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. Chemistry Definition Of Calorimeter.
From themasterchemistry.com
Calorimeter Types, Principle, Working, Uses Chemistry Definition Of Calorimeter Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device. Chemistry Definition Of Calorimeter.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter. Chemistry Definition Of Calorimeter.
From www.youtube.com
Calorific value check for petroleum Oil Through the help of bomb Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the. Chemistry Definition Of Calorimeter.
From www.animalia-life.club
Calorimeter Diagram Chemistry Definition Of Calorimeter In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. By knowing the change in heat, it can be. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat. Chemistry Definition Of Calorimeter.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry Chemistry Definition Of Calorimeter For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and. Chemistry Definition Of Calorimeter.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in heat, it can be. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Chemistry Definition Of Calorimeter.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Chemistry Definition Of Calorimeter Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in heat, it can be. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical. Chemistry Definition Of Calorimeter.
From socratic.org
What is the change in internal energy of reaction when "0.721 g" of Chemistry Definition Of Calorimeter For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimetry is. Chemistry Definition Of Calorimeter.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For. Chemistry Definition Of Calorimeter.
From martindxmguide.blogspot.com
32 Chemistry Worksheet Heat And Calorimetry Problems support worksheet Chemistry Definition Of Calorimeter For example, when an exothermic. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimetry is the process of measuring the amount of heat released or absorbed during. Chemistry Definition Of Calorimeter.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Chemistry Definition Of Calorimeter Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials.. Chemistry Definition Of Calorimeter.
From jacyou.com
モル熱容量とは何ですか?どのように計算しますか? 世界地図 Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount of heat involved in a. Chemistry Definition Of Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Chemistry Definition Of Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an exothermic. For. Chemistry Definition Of Calorimeter.
From printablecampusreises.z21.web.core.windows.net
How To Solve Calorimetry Problems Chemistry Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure'). Chemistry Definition Of Calorimeter.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Chemistry Definition Of Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A. Chemistry Definition Of Calorimeter.
From loerzgmcm.blob.core.windows.net
How To Make A Homemade Calorimeter at Norma Mercer blog Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the heat flow of a chemical reaction. Chemistry Definition Of Calorimeter.
From dxodvsdxa.blob.core.windows.net
Calorimeter In Physics Meaning at Dennis Flores blog Chemistry Definition Of Calorimeter In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By. Chemistry Definition Of Calorimeter.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Chemistry Definition Of Calorimeter In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter. Chemistry Definition Of Calorimeter.
From cernvbyt.blob.core.windows.net
Can Heat Capacity Of Calorimeter Be Negative at Lulu Seaton blog Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in heat, it can be. For example, when an exothermic. A calorimeter is a device used to measure. Chemistry Definition Of Calorimeter.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Chemistry Definition Of Calorimeter For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. By knowing the change in heat, it can be. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to. Chemistry Definition Of Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Chemistry Definition Of Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. By knowing the change in heat, it can be. Calorimetry is the process of measuring the amount of heat released or absorbed. Chemistry Definition Of Calorimeter.
From loevclgsu.blob.core.windows.net
Calorimeter Thermochemistry Definition at Leona Fraher blog Chemistry Definition Of Calorimeter By knowing the change in heat, it can be. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the heat flow. Chemistry Definition Of Calorimeter.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Chemistry Definition Of Calorimeter By knowing the change in heat, it can be. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat. Chemistry Definition Of Calorimeter.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Chemistry Definition Of Calorimeter For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor. Chemistry Definition Of Calorimeter.
From loejpndsk.blob.core.windows.net
Calorimeter Uncertainty at Nancy Conger blog Chemistry Definition Of Calorimeter Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. In chemistry and thermodynamics, calorimetry. Chemistry Definition Of Calorimeter.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Chemistry Definition Of Calorimeter In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an exothermic. Calorimetry is. Chemistry Definition Of Calorimeter.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Chemistry Definition Of Calorimeter For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released. Chemistry Definition Of Calorimeter.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Chemistry Definition Of Calorimeter By knowing the change in heat, it can be. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction. Chemistry Definition Of Calorimeter.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Chemistry Definition Of Calorimeter Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimeter, device for measuring the heat developed during a mechanical,. Chemistry Definition Of Calorimeter.
From www.animalia-life.club
Calorimeter Diagram Chemistry Definition Of Calorimeter For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the heat flow of a chemical reaction or. Chemistry Definition Of Calorimeter.