Lab Kit Destruction Log . O the responsible study team member should identify what will be done with the unused drug,. Destruction of investigational products author: Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Return/destruction portion of the log as follows:
from www.bestmedicalforms.com
1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Return/destruction portion of the log as follows: Destruction of investigational products author: O the responsible study team member should identify what will be done with the unused drug,. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer.
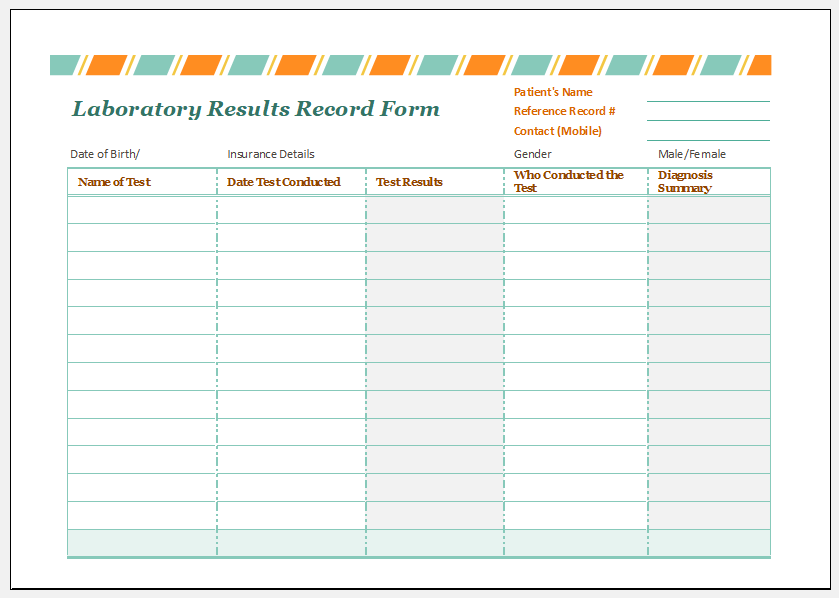
Laboratory Results Record Form Template Download
Lab Kit Destruction Log 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). Destruction of investigational products author: 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. O the responsible study team member should identify what will be done with the unused drug,. Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Return/destruction portion of the log as follows: Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a.
From www.dexform.com
Sanitizer test strip log in Word and Pdf formats Lab Kit Destruction Log All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). O the responsible study team member should identify what will be done with the unused. Lab Kit Destruction Log.
From www.dochub.com
Medication destruction form Fill out & sign online DocHub Lab Kit Destruction Log Destruction of investigational products author: All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. O the responsible study team member should identify what. Lab Kit Destruction Log.
From www.carousell.ph
Complete laboratory kit lab kit, Furniture & Home Living, Cleaning Lab Kit Destruction Log All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. O the responsible study team member should identify what will be done with the unused drug,. Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior. Lab Kit Destruction Log.
From pharmacyequipmentmarketplace.com
Controlled Substance Waste Log RxMarketplace Lab Kit Destruction Log All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and. Lab Kit Destruction Log.
From pharmaceuticalupdates.com
Equipment Usage LogbookProcedure and Format Pharmaceutical Updates Lab Kit Destruction Log Return/destruction portion of the log as follows: Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. 1.1 this procedure describes steps for the destruction of laboratory specimens from. Lab Kit Destruction Log.
From www.pinterest.ph
the certificate for records destruction is shown in this document Lab Kit Destruction Log Return/destruction portion of the log as follows: Destruction of investigational products author: All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. 1.1 this procedure describes steps for the destruction of laboratory specimens. Lab Kit Destruction Log.
From uob-cay.blogspot.com
Free Printable Controlled Substance Log Lab Kit Destruction Log Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Destruction of investigational products author: 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious. Lab Kit Destruction Log.
From qualitycompliance.research.utah.edu
Tool Kit Research Quality and Compliance The University of Utah Lab Kit Destruction Log Destruction of investigational products author: Return/destruction portion of the log as follows: Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. 1.1 this procedure describes steps for the. Lab Kit Destruction Log.
From www.dexform.com
Laboratory specimen tracking form in Word and Pdf formats Lab Kit Destruction Log O the responsible study team member should identify what will be done with the unused drug,. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. Destruction of investigational products author: Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior. Lab Kit Destruction Log.
From www.mrs-scientific.com
LABORATORY SPILL KIT MERCURY MRS Scientific Lab Kit Destruction Log Destruction of investigational products author: 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Provide the specimen lab kit to the crc clinical research. Lab Kit Destruction Log.
From www.dreamstime.com
Emergency Spill Kit Wall Signs in Box for Use in Laboratory in T Stock Lab Kit Destruction Log O the responsible study team member should identify what will be done with the unused drug,. Return/destruction portion of the log as follows: All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Provide the specimen lab kit to the crc clinical research associates on the day of the. Lab Kit Destruction Log.
From ekdoseispelasgos.blogspot.com
Food Waste Log Template Master Template Lab Kit Destruction Log 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). O the responsible study team member should identify what will be done with the unused drug,. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. Return/destruction portion of the. Lab Kit Destruction Log.
From jo-anngarbutt.org
Training Class Signin sheet Recycled Lab Kit Destruction Log 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. O the responsible study team member should identify what will be done with the unused. Lab Kit Destruction Log.
From www.process.st
Records Disposal Checklist Process Street Lab Kit Destruction Log 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). O the responsible study team member should identify what will be done with the unused drug,. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. Destruction of investigational products. Lab Kit Destruction Log.
From www.bestmedicalforms.com
Laboratory Results Record Form Template Download Lab Kit Destruction Log All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute. Lab Kit Destruction Log.
From lesboucans.com
Quality Control Log Template Database Lab Kit Destruction Log Destruction of investigational products author: 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). Return/destruction portion of the log as follows: O the responsible study team member should identify what will be done with the unused drug,. All clinical trials materials and investigational medicinal products. Lab Kit Destruction Log.
From www.flinnsci.com
Advanced SlideMaking—Student Laboratory Kit Flinn Scientific Lab Kit Destruction Log O the responsible study team member should identify what will be done with the unused drug,. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). Destruction of investigational products author: All clinical trials materials and investigational medicinal products (imp) that have been used, partially used,. Lab Kit Destruction Log.
From www.pinterest.com
Laboratory Survival Kit Tag Laboratory Medical Gifts Lab Tech Gift Lab Kit Destruction Log Destruction of investigational products author: Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Return/destruction portion of the log as follows: Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. All clinical trials materials and investigational medicinal. Lab Kit Destruction Log.
From shop.oakmeadow.com
Chemistry Matters Lab Kit Set Oak Meadow Lab Kit Destruction Log All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. O the responsible study team member should identify what will be done with the unused drug,. Provide the specimen lab kit to the. Lab Kit Destruction Log.
From mungfali.com
Dental Lab Log Sheet Printable Lab Kit Destruction Log Return/destruction portion of the log as follows: 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). O the responsible study team member should identify what will be done with the unused drug,. Destruction for all expired medications will be documented in the ids accountability system,. Lab Kit Destruction Log.
From www.oakmeadowbookstore.com
Chemistry Matters Lab Kit Set Oak Meadow Bookstore Lab Kit Destruction Log Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids. Lab Kit Destruction Log.
From www.etsy.com
Printable Lab Work Sheet Blood Work Log Medical Test Record Etsy Israel Lab Kit Destruction Log All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. Destruction of investigational products author: O the responsible study team member should identify what will be done with the unused drug,. Return/destruction portion. Lab Kit Destruction Log.
From www.templateroller.com
Form DOC01089 Download Printable PDF or Fill Online Records Lab Kit Destruction Log Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). All clinical trials materials and investigational medicinal products (imp) that have. Lab Kit Destruction Log.
From www.rainbowresource.com
Lab Kit for use with Abeka Science Grade 7 Home Science Tools Lab Kit Destruction Log Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. Return/destruction portion of the log as follows: O the responsible study team member should identify what will be done with the unused drug,. Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined. Lab Kit Destruction Log.
From www.breathalyzer.co.uk
Drug Test Urine Laboratory Confirmation Kit 10 Drug Types AlcoDigital Lab Kit Destruction Log Return/destruction portion of the log as follows: 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Destruction of investigational products. Lab Kit Destruction Log.
From taylormadescience.com
Lab Supply Kits Taylor Made Science Lab Kit Destruction Log Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. O the responsible study team member should identify what will be done with the unused drug,. Return/destruction portion of the log as follows: Destruction of investigational products author: Provide the specimen lab kit to the crc clinical research associates on the day of the. Lab Kit Destruction Log.
From mavink.com
Medication Destruction Log Sheet Printable Lab Kit Destruction Log Return/destruction portion of the log as follows: O the responsible study team member should identify what will be done with the unused drug,. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). Destruction for all expired medications will be documented in the ids accountability system,. Lab Kit Destruction Log.
From taylormadescience.com
Lab Supply Kits Taylor Made Science Lab Kit Destruction Log 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Destruction of investigational products author: Provide the specimen lab kit to the crc clinical research. Lab Kit Destruction Log.
From www.carousell.ph
Laboratory Kit on Carousell Lab Kit Destruction Log Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Return/destruction portion of the log as follows: Destruction of investigational products author: All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. Destruction for. Lab Kit Destruction Log.
From paulprintable.com
Free Printable Controlled Substance Log Free Printable Lab Kit Destruction Log Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. Destruction of investigational products author: O the responsible study team member should identify what will be done with the unused drug,. Return/destruction portion of the log as follows: 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy. Lab Kit Destruction Log.
From www.homesciencetools.com
Christian Light Grade 9 Science Lab Kit HST Lab Kit Destruction Log Return/destruction portion of the log as follows: Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. 1.1 this procedure describes steps for the destruction of laboratory specimens from. Lab Kit Destruction Log.
From klamvacyb.blob.core.windows.net
Log Book Reference Number at Pauline Mitchell blog Lab Kit Destruction Log Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are. Lab Kit Destruction Log.
From www.labtestsguide.com
Best Practices for Managing Laboratory Waste 6 Tips and Guidelines Lab Kit Destruction Log All clinical trials materials and investigational medicinal products (imp) that have been used, partially used, or unused but are no longer. O the responsible study team member should identify what will be done with the unused drug,. Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior. Lab Kit Destruction Log.
From www.studocu.com
CHM 116 Lab Kit Inventory Open your lab kit and remove all of the Lab Kit Destruction Log 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division of aids (daids). Provide the specimen lab kit to the crc clinical research associates on the day of the visit, or at a predetermined time prior to the. Destruction of investigational products author: Return/destruction portion of the log as. Lab Kit Destruction Log.
From www.flinnsci.com
Flinn Advanced Laboratory Kits for AP* Environmental Science 14Kit Bundle Lab Kit Destruction Log Destruction for all expired medications will be documented in the ids accountability system, vestigo®, and a. Return/destruction portion of the log as follows: O the responsible study team member should identify what will be done with the unused drug,. 1.1 this procedure describes steps for the destruction of laboratory specimens from national institute of allergy and infectious diseases (niaid) division. Lab Kit Destruction Log.