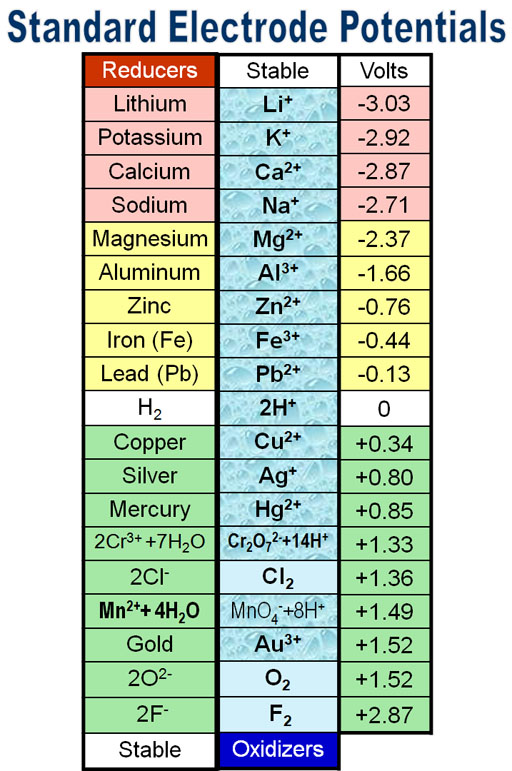
Standard Reduction Potential By Element . The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The values of standard electrode potentials are given in the table in. F standard state of elements; Table of standard reduction potentials (modified from wikipedia). E ° is the standard reduction potential. Reduction reactions in acidic solution are written using h + in place of h 3 o +. 183 rows the following table provides e o for selected reduction reactions. H standard reduction potentials (by name) i. G standard thermodynamic values for select substances; Values are from the following sources: You may rewrite a reaction by replacing h + with h 3 o + and adding. The more positive the potential is the.
from budgetlightforum.com
G standard thermodynamic values for select substances; The more positive the potential is the. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). Values are from the following sources: Reduction reactions in acidic solution are written using h + in place of h 3 o +. 183 rows the following table provides e o for selected reduction reactions. Table of standard reduction potentials (modified from wikipedia). E ° is the standard reduction potential. H standard reduction potentials (by name) i. F standard state of elements;
Batteries
Standard Reduction Potential By Element Reduction reactions in acidic solution are written using h + in place of h 3 o +. Reduction reactions in acidic solution are written using h + in place of h 3 o +. H standard reduction potentials (by name) i. 183 rows the following table provides e o for selected reduction reactions. The more positive the potential is the. Values are from the following sources: Table of standard reduction potentials (modified from wikipedia). E ° is the standard reduction potential. F standard state of elements; The values of standard electrode potentials are given in the table in. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). You may rewrite a reaction by replacing h + with h 3 o + and adding. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. G standard thermodynamic values for select substances;
From www.chegg.com
Solved Refer to the table of standard reduction potentials Standard Reduction Potential By Element H standard reduction potentials (by name) i. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). Values are from the following sources: The more positive the potential is the. The values of standard electrode potentials are given in the table in. F standard state of elements; E ° is. Standard Reduction Potential By Element.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential By Element You may rewrite a reaction by replacing h + with h 3 o + and adding. E ° is the standard reduction potential. H standard reduction potentials (by name) i. G standard thermodynamic values for select substances; Table of standard reduction potentials (modified from wikipedia). The standard reduction potential is the tendency for a chemical species to be reduced, and. Standard Reduction Potential By Element.
From www.toppr.com
Using the standard electrode potentials given in the table, predict the Standard Reduction Potential By Element H standard reduction potentials (by name) i. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). The more positive the potential is the. The values of standard electrode potentials are given in the table in. Values are from the following sources: 183 rows the following table provides e o. Standard Reduction Potential By Element.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential By Element Table of standard reduction potentials (modified from wikipedia). The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). F standard state of elements; E ° is the standard reduction potential. Values are from the following sources: H standard reduction potentials (by name) i. You may rewrite a reaction by replacing. Standard Reduction Potential By Element.
From www.numerade.com
SOLVED Standard Reduction Potentials at 25^∘C^* Copyright (c) The Standard Reduction Potential By Element Table of standard reduction potentials (modified from wikipedia). F standard state of elements; Values are from the following sources: The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. H standard reduction potentials (by name) i. 183 rows the following table provides e o for selected reduction reactions.. Standard Reduction Potential By Element.
From byjus.com
Is oxidation potential always positive and reduction potential always Standard Reduction Potential By Element The more positive the potential is the. F standard state of elements; The values of standard electrode potentials are given in the table in. Values are from the following sources: 183 rows the following table provides e o for selected reduction reactions. Table of standard reduction potentials (modified from wikipedia). The standard reduction potential is the tendency for a chemical. Standard Reduction Potential By Element.
From exoqlpkuh.blob.core.windows.net
Standard Cell Potential Chart at Ada Cottone blog Standard Reduction Potential By Element Values are from the following sources: G standard thermodynamic values for select substances; The more positive the potential is the. Reduction reactions in acidic solution are written using h + in place of h 3 o +. H standard reduction potentials (by name) i. 183 rows the following table provides e o for selected reduction reactions. F standard state of. Standard Reduction Potential By Element.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential By Element Table of standard reduction potentials (modified from wikipedia). Reduction reactions in acidic solution are written using h + in place of h 3 o +. E ° is the standard reduction potential. The values of standard electrode potentials are given in the table in. The standard reduction potential is the tendency for a chemical species to be reduced, and is. Standard Reduction Potential By Element.
From www.researchgate.net
Reduction potentials of redox couple elements and potential auxiliary Standard Reduction Potential By Element H standard reduction potentials (by name) i. The more positive the potential is the. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Table of standard reduction potentials (modified from wikipedia). 183 rows the following table provides e o for selected reduction reactions. F standard state of elements; You may rewrite a. Standard Reduction Potential By Element.
From www.chegg.com
Solved Use the standard reduction potentials located in the Standard Reduction Potential By Element Values are from the following sources: The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for. Standard Reduction Potential By Element.
From question.pandai.org
Standard Electrode Potential Standard Reduction Potential By Element E ° is the standard reduction potential. Reduction reactions in acidic solution are written using h + in place of h 3 o +. H standard reduction potentials (by name) i. Table of standard reduction potentials (modified from wikipedia). The more positive the potential is the. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm. Standard Reduction Potential By Element.
From budgetlightforum.com
Batteries Standard Reduction Potential By Element Reduction reactions in acidic solution are written using h + in place of h 3 o +. G standard thermodynamic values for select substances; The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). Table of standard reduction potentials (modified from wikipedia). Values are from the following sources: The more. Standard Reduction Potential By Element.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential By Element The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The values of standard electrode potentials are given in the table in. 183 rows the following table provides e o for selected reduction reactions. F standard state of elements; H standard reduction potentials (by name) i. The superscript. Standard Reduction Potential By Element.
From www.pinterest.com
Standard Reduction Potential (E) when given two half reactions and Standard Reduction Potential By Element G standard thermodynamic values for select substances; Values are from the following sources: Table of standard reduction potentials (modified from wikipedia). E ° is the standard reduction potential. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). H standard reduction potentials (by name) i. You may rewrite a reaction. Standard Reduction Potential By Element.
From chemistrytalk.org
Standard Reduction Potentials made easy ChemTalk Standard Reduction Potential By Element The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Values are from the following sources: Table of standard reduction potentials (modified from wikipedia). Reduction reactions in acidic solution are written using h + in place of h 3 o +. The superscript “°” on the e denotes. Standard Reduction Potential By Element.
From www.vedantu.com
Oxidation Potential and Number Important Concepts and Tips for JEE Standard Reduction Potential By Element Table of standard reduction potentials (modified from wikipedia). 183 rows the following table provides e o for selected reduction reactions. Values are from the following sources: The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). The values of standard electrode potentials are given in the table in. You may. Standard Reduction Potential By Element.
From www.slideserve.com
PPT Determine the oxidation number for each atom in the following Standard Reduction Potential By Element F standard state of elements; E ° is the standard reduction potential. You may rewrite a reaction by replacing h + with h 3 o + and adding. The more positive the potential is the. H standard reduction potentials (by name) i. 183 rows the following table provides e o for selected reduction reactions. The superscript “°” on the e. Standard Reduction Potential By Element.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential By Element E ° is the standard reduction potential. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Table of standard reduction potentials (modified from wikipedia). The more positive the potential is the. H standard reduction potentials (by name) i. Reduction reactions in acidic solution are written using h. Standard Reduction Potential By Element.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Reduction Potential By Element You may rewrite a reaction by replacing h + with h 3 o + and adding. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The superscript “°” on the. Standard Reduction Potential By Element.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Standard Reduction Potential By Element F standard state of elements; G standard thermodynamic values for select substances; Table of standard reduction potentials (modified from wikipedia). The more positive the potential is the. The values of standard electrode potentials are given in the table in. 183 rows the following table provides e o for selected reduction reactions. E ° is the standard reduction potential. H standard. Standard Reduction Potential By Element.
From exovxcvqo.blob.core.windows.net
Standard Reduction Potential Gold at Juan Delk blog Standard Reduction Potential By Element G standard thermodynamic values for select substances; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Values are from the following sources: E ° is the standard reduction potential. The values of standard electrode potentials are given in the table in. H standard reduction potentials (by name). Standard Reduction Potential By Element.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential By Element Table of standard reduction potentials (modified from wikipedia). 183 rows the following table provides e o for selected reduction reactions. The values of standard electrode potentials are given in the table in. The more positive the potential is the. F standard state of elements; The standard reduction potential is the tendency for a chemical species to be reduced, and is. Standard Reduction Potential By Element.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free Standard Reduction Potential By Element The values of standard electrode potentials are given in the table in. Values are from the following sources: You may rewrite a reaction by replacing h + with h 3 o + and adding. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). The standard reduction potential is the. Standard Reduction Potential By Element.
From exovxcvqo.blob.core.windows.net
Standard Reduction Potential Gold at Juan Delk blog Standard Reduction Potential By Element 183 rows the following table provides e o for selected reduction reactions. Values are from the following sources: H standard reduction potentials (by name) i. Reduction reactions in acidic solution are written using h + in place of h 3 o +. G standard thermodynamic values for select substances; The more positive the potential is the. The superscript “°” on. Standard Reduction Potential By Element.
From www.scribd.com
P1 Standard Reduction Potentials by Element PDF Manganese Chemistry Standard Reduction Potential By Element E ° is the standard reduction potential. Values are from the following sources: 183 rows the following table provides e o for selected reduction reactions. The more positive the potential is the. You may rewrite a reaction by replacing h + with h 3 o + and adding. H standard reduction potentials (by name) i. Table of standard reduction potentials. Standard Reduction Potential By Element.
From dxoqcxjmj.blob.core.windows.net
Standard Reduction Potential Example at Toni Officer blog Standard Reduction Potential By Element You may rewrite a reaction by replacing h + with h 3 o + and adding. The values of standard electrode potentials are given in the table in. The more positive the potential is the. Table of standard reduction potentials (modified from wikipedia). G standard thermodynamic values for select substances; E ° is the standard reduction potential. Values are from. Standard Reduction Potential By Element.
From protonstalk.com
Electrochemical Series Features, Applications, Examples ProtonsTalk Standard Reduction Potential By Element H standard reduction potentials (by name) i. The values of standard electrode potentials are given in the table in. G standard thermodynamic values for select substances; Reduction reactions in acidic solution are written using h + in place of h 3 o +. 183 rows the following table provides e o for selected reduction reactions. The superscript “°” on the. Standard Reduction Potential By Element.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential By Element G standard thermodynamic values for select substances; The more positive the potential is the. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Values are from the following sources: Table of standard reduction potentials (modified from wikipedia). F standard state of elements; H standard reduction potentials (by. Standard Reduction Potential By Element.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential By Element H standard reduction potentials (by name) i. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Values are from the following sources: The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The more positive the potential is the. The. Standard Reduction Potential By Element.
From www.chegg.com
Solved Using standard reduction potentials from the ALEKS Standard Reduction Potential By Element The values of standard electrode potentials are given in the table in. Table of standard reduction potentials (modified from wikipedia). The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). F standard state of elements; G standard thermodynamic values for select substances; E ° is the standard reduction potential. Values. Standard Reduction Potential By Element.
From www.researchgate.net
Standard reduction potential (V vs Ag/AgCl) of different elements in Standard Reduction Potential By Element You may rewrite a reaction by replacing h + with h 3 o + and adding. The more positive the potential is the. 183 rows the following table provides e o for selected reduction reactions. E ° is the standard reduction potential. Values are from the following sources: The values of standard electrode potentials are given in the table in.. Standard Reduction Potential By Element.
From www.researchgate.net
1 Standard redox potentials involving lanthanide elements as dissolved Standard Reduction Potential By Element Reduction reactions in acidic solution are written using h + in place of h 3 o +. Values are from the following sources: E ° is the standard reduction potential. Table of standard reduction potentials (modified from wikipedia). The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions.. Standard Reduction Potential By Element.
From www.pinterest.com
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Standard Reduction Potential By Element 183 rows the following table provides e o for selected reduction reactions. G standard thermodynamic values for select substances; H standard reduction potentials (by name) i. Table of standard reduction potentials (modified from wikipedia). Values are from the following sources: Reduction reactions in acidic solution are written using h + in place of h 3 o +. The superscript “°”. Standard Reduction Potential By Element.
From dxoasuand.blob.core.windows.net
Calculate The Standard Cell Potentials Given The Following Data at Standard Reduction Potential By Element Table of standard reduction potentials (modified from wikipedia). H standard reduction potentials (by name) i. The values of standard electrode potentials are given in the table in. G standard thermodynamic values for select substances; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. E ° is the. Standard Reduction Potential By Element.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential By Element You may rewrite a reaction by replacing h + with h 3 o + and adding. G standard thermodynamic values for select substances; Table of standard reduction potentials (modified from wikipedia). H standard reduction potentials (by name) i. The superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). Values are. Standard Reduction Potential By Element.