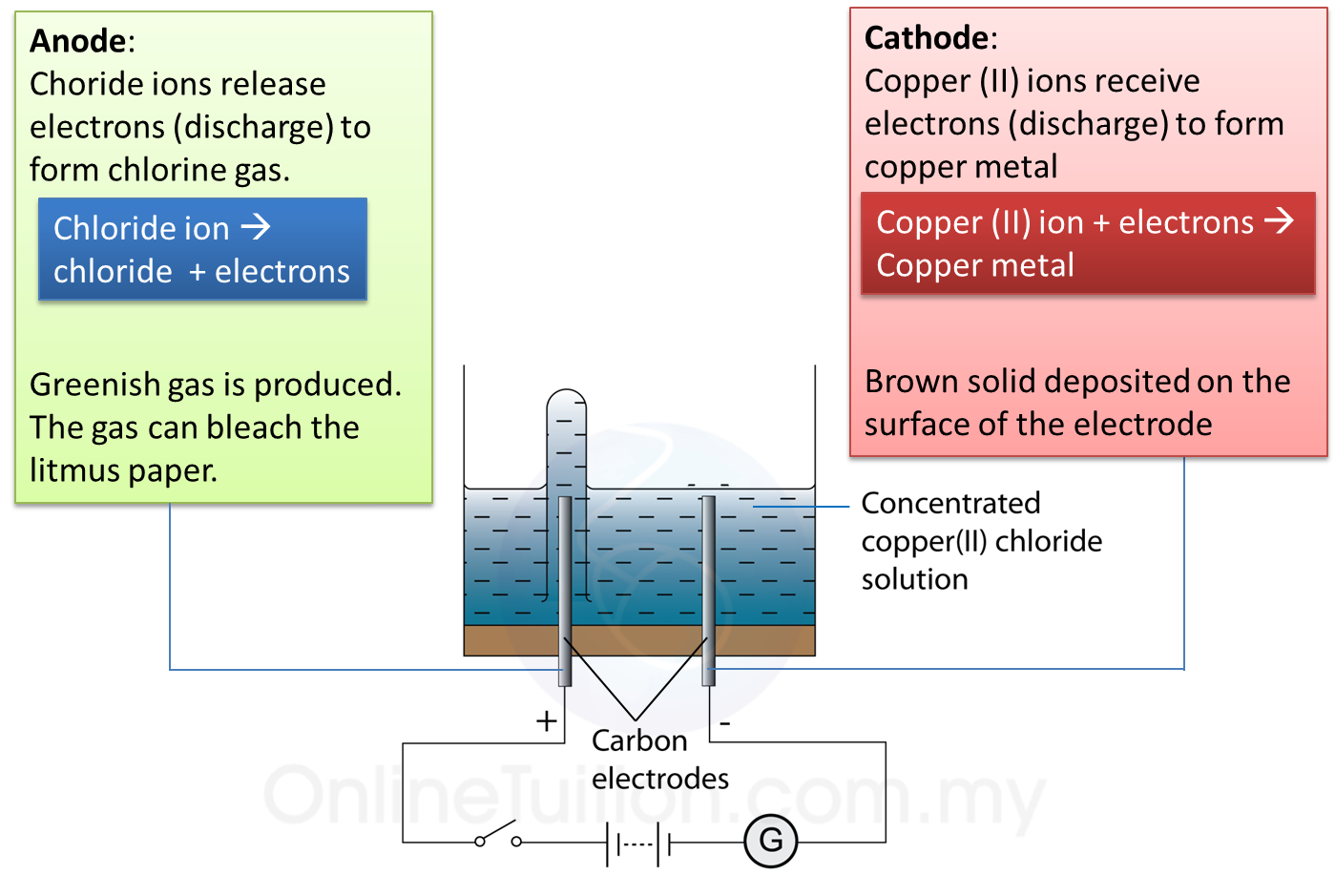
Copper Ii Chloride And Sodium Hydroxide Balanced Equation . — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Ap®︎/college chemistry > unit 4. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Molecular, complete ionic, and net ionic equations. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water.
from content.myhometuition.com
Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry > unit 4. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,.
Energy and Chemical Changes user's Blog!
Copper Ii Chloride And Sodium Hydroxide Balanced Equation diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Ap®︎/college chemistry > unit 4. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. Molecular, complete ionic, and net ionic equations. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Copper Ii Chloride And Sodium Hydroxide Balanced Equation — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Molecular, complete ionic, and net ionic equations. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. learn how to balance chemical equations using the law of conservation of mass and the method of. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED what happens when sodium hydroxide reacts with sulphuric acid Copper Ii Chloride And Sodium Hydroxide Balanced Equation — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. Molecular, complete ionic, and net ionic equations. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. learn how. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.youtube.com
How to Balance CuCl2 + NaOH = Cu(OH)2 + NaCl Copper (II) chloride Copper Ii Chloride And Sodium Hydroxide Balanced Equation — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. Molecular, complete ionic, and net ionic equations. diatomic. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED 14.Write the equilibrium constant expression for the Copper Ii Chloride And Sodium Hydroxide Balanced Equation Ap®︎/college chemistry > unit 4. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. Molecular, complete ionic, and net ionic equations. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. — in this video we will balance the equation cucl2 + naoh. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From basdemax.netlify.app
28++ Hydrochloric Acid And Sodium Hydroxide Balanced Equation Basdemax Copper Ii Chloride And Sodium Hydroxide Balanced Equation Molecular, complete ionic, and net ionic equations. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. Ap®︎/college chemistry > unit 4. learn how to balance chemical equations using the law of conservation of mass. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Copper Ii Chloride And Sodium Hydroxide Balanced Equation — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry > unit 4. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation Molecular, complete ionic, and net ionic equations. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,.. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From ar.inspiredpencil.com
Copper Ii Chloride Lewis Structure Copper Ii Chloride And Sodium Hydroxide Balanced Equation — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Molecular, complete ionic, and net ionic. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From content.myhometuition.com
Energy and Chemical Changes user's Blog! Copper Ii Chloride And Sodium Hydroxide Balanced Equation diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to balance chemical equations. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From dxoayxwhw.blob.core.windows.net
Bromine Sodium Hydroxide Balanced Equation at Lisa Swearengin blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. Ap®︎/college chemistry > unit 4. Molecular, complete ionic, and net ionic equations. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From asideload7.gitlab.io
Formidable Word Equation Of Sodium Chloride Edexcel Physics Syllabus Copper Ii Chloride And Sodium Hydroxide Balanced Equation Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. diatomic chlorine and sodium hydroxide. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From insende.netlify.app
30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende Copper Ii Chloride And Sodium Hydroxide Balanced Equation Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry > unit 4. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. learn how to balance chemical equations using the law of conservation of mass and the. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From hxelaptcw.blob.core.windows.net
Copper Ii Sulfate Sodium Phosphate Balanced Equation at Raymond Horan blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Molecular, complete ionic, and net ionic equations. — use. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From hxelaptcw.blob.core.windows.net
Copper Ii Sulfate Sodium Phosphate Balanced Equation at Raymond Horan blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. Molecular, complete ionic, and net ionic equations. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients.. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Copper Ii Chloride And Sodium Hydroxide Balanced Equation — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. — in this. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From exovhomju.blob.core.windows.net
Copper (Ii) Chloride And Sodium Hydroxide Reaction at Marie Croom blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Ap®︎/college chemistry > unit 4. Cupric chloride. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952 Copper Ii Chloride And Sodium Hydroxide Balanced Equation Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry > unit 4. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. — in this video we will balance the equation cucl2. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From oneclass.com
OneClass Write the balanced net ionic equation for the reactions that Copper Ii Chloride And Sodium Hydroxide Balanced Equation — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Ap®︎/college chemistry > unit 4. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. Molecular, complete. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Copper Ii Chloride And Sodium Hydroxide Balanced Equation diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. — use either a net ionic. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From solvedlib.com
Write net ionic equations for the reaction, if any, t… SolvedLib Copper Ii Chloride And Sodium Hydroxide Balanced Equation — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. learn how to balance chemical equations. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From chemistry.stackexchange.com
experimental chemistry Reaction between copper (II)chloride and Copper Ii Chloride And Sodium Hydroxide Balanced Equation — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Molecular, complete ionic, and net ionic equations. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. —. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.youtube.com
Sodium hydroxide and ammonia added to copper (II) chloride solution Copper Ii Chloride And Sodium Hydroxide Balanced Equation Ap®︎/college chemistry > unit 4. Molecular, complete ionic, and net ionic equations. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. — in this video we will balance the equation cucl2 + naoh =. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Water And Sodium Hydroxide Tessshebaylo Copper Ii Chloride And Sodium Hydroxide Balanced Equation diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Ap®︎/college chemistry > unit 4. Molecular, complete ionic, and net ionic equations. — in this video we will balance the equation cucl2. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From dxoayxwhw.blob.core.windows.net
Bromine Sodium Hydroxide Balanced Equation at Lisa Swearengin blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Ii Chloride And Sodium Hydroxide Balanced Equation Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Ap®︎/college chemistry > unit 4. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. diatomic chlorine and. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From insende.netlify.app
30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende Copper Ii Chloride And Sodium Hydroxide Balanced Equation Molecular, complete ionic, and net ionic equations. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. Ap®︎/college chemistry > unit 4. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From studychronicled.z21.web.core.windows.net
Net Ionic Equation For Hcl And Naoh Copper Ii Chloride And Sodium Hydroxide Balanced Equation — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. diatomic chlorine and sodium hydroxide. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.slideshare.net
Chemical Reactions Copper Ii Chloride And Sodium Hydroxide Balanced Equation diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. — use either a net ionic equations (omit the. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation Molecular, complete ionic, and net ionic equations. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Ap®︎/college chemistry > unit 4. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. — use either a net ionic equations (omit the na+),. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for CoCl2 + NaOH = Co(OH)2 + NaCl Copper Ii Chloride And Sodium Hydroxide Balanced Equation Ap®︎/college chemistry > unit 4. Molecular, complete ionic, and net ionic equations. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. learn how to balance chemical equations using the law of. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From giolompon.blob.core.windows.net
Iron Iii Chloride Sodium Hydroxide Net Ionic Equation at Fran Swain blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Ap®︎/college chemistry > unit 4. learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. . Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry > unit 4. — use either a net ionic equations (omit the na+),. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From exovhomju.blob.core.windows.net
Copper (Ii) Chloride And Sodium Hydroxide Reaction at Marie Croom blog Copper Ii Chloride And Sodium Hydroxide Balanced Equation Molecular, complete ionic, and net ionic equations. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Ap®︎/college chemistry > unit 4. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. — in this video we will balance the equation cucl2. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED write balanced chemical equation between oxalic acid and sodium Copper Ii Chloride And Sodium Hydroxide Balanced Equation — in this video we will balance the equation cucl2 + naoh = cu(oh)2 +. — use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Molecular, complete ionic, and net ionic equations.. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Question 9 solid sodium chloride is produced by the reaction of Copper Ii Chloride And Sodium Hydroxide Balanced Equation Molecular, complete ionic, and net ionic equations. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Ap®︎/college chemistry > unit 4. Cupric chloride + sodium hydroxide + hydrogen peroxide = copper(ii) oxide + sodium chloride + water. — in this video we will balance the equation cucl2 + naoh =. Copper Ii Chloride And Sodium Hydroxide Balanced Equation.