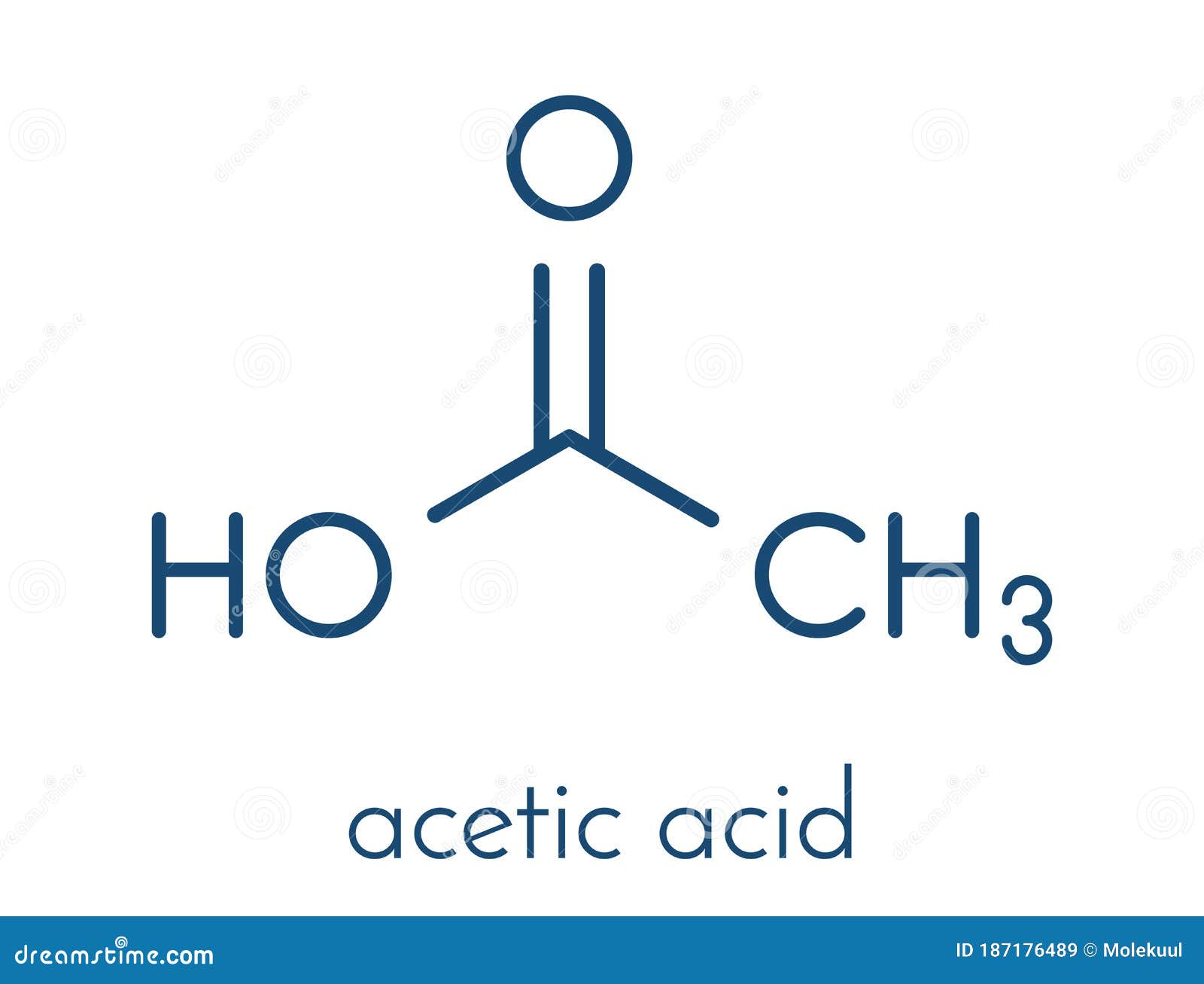
Vinegar Acid Weak . the molecular formula for water is h 2 o. If you dilute vinegar with water, its acidity lessens, making. In case of contact with a strong. what acid is in vinegar? vinegar is often considered a weak acid due to its composition of acetic acid. The structural formula for acetic acid is ch 3 cooh. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. This acidity gives vinegar its distinct sour taste and is. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same.
from www.dreamstime.com
vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. This acidity gives vinegar its distinct sour taste and is. vinegar is often considered a weak acid due to its composition of acetic acid. you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. The structural formula for acetic acid is ch 3 cooh. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. the molecular formula for water is h 2 o. If you dilute vinegar with water, its acidity lessens, making. what acid is in vinegar? In case of contact with a strong.
Acetic Acid Molecule. Vinegar is an Aqueous Solution of Acetic Acid
Vinegar Acid Weak you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. In case of contact with a strong. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. This acidity gives vinegar its distinct sour taste and is. the molecular formula for water is h 2 o. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. vinegar is often considered a weak acid due to its composition of acetic acid. The structural formula for acetic acid is ch 3 cooh. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. If you dilute vinegar with water, its acidity lessens, making. you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. what acid is in vinegar?
From www.allaboutsmilesde.com
What is Oral pH and How Does it Affect Your Health? All About Smiles Vinegar Acid Weak In case of contact with a strong. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. vinegar is often considered a weak acid due to its composition of acetic acid. The structural formula for acetic acid is ch 3 cooh. the molecular formula for water is h 2 o. If. Vinegar Acid Weak.
From slideplayer.com
Conjugate acids and bases ppt download Vinegar Acid Weak the molecular formula for water is h 2 o. The structural formula for acetic acid is ch 3 cooh. If you dilute vinegar with water, its acidity lessens, making. This acidity gives vinegar its distinct sour taste and is. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in. Vinegar Acid Weak.
From www.numerade.com
SOLVEDVinegar contains acetic acid, a weak acid that is partially Vinegar Acid Weak you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. If you dilute vinegar with water, its acidity lessens, making. vinegar, being a weak acid, can help neutralize strong bases rather. Vinegar Acid Weak.
From www.compoundchem.com
Compound Interest The sour science of vinegar varieties Vinegar Acid Weak this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. what acid is in vinegar? vinegar is often considered a weak acid due to its composition of acetic acid. the molecular formula for water is h 2 o. you can drink diluted acetic acid. Vinegar Acid Weak.
From eatforlonger.com
What Is The Most Acidic Vinegar? Eat For Longer Food Insights Vinegar Acid Weak what acid is in vinegar? this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. In case of contact with a strong. This acidity gives vinegar its distinct sour taste and is. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids.. Vinegar Acid Weak.
From learningdbhaughtier.z13.web.core.windows.net
What Is The Ph Level Of Battery Acid Vinegar Acid Weak The structural formula for acetic acid is ch 3 cooh. what acid is in vinegar? you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. In case of contact with a. Vinegar Acid Weak.
From studiousguy.com
Acetic Acid Uses in Daily Life StudiousGuy Vinegar Acid Weak you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. This acidity gives vinegar its distinct sour taste and is. If you dilute vinegar with water, its acidity lessens, making. The structural formula for acetic acid is ch 3 cooh. this is the weakest level of acidity in vinegar, and is not legal. Vinegar Acid Weak.
From exoweudjm.blob.core.windows.net
How To Check Ph Value Of Drinking Water At Home at Lynn Reif blog Vinegar Acid Weak what acid is in vinegar? you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. The structural formula for acetic acid is ch 3 cooh. In case of contact with a strong. the molecular formula for water. Vinegar Acid Weak.
From printablegeminigirl83my.z4.web.core.windows.net
How To Identify Acids And Bases In A Equation Vinegar Acid Weak vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. the molecular formula for water is h 2 o. vinegar is often considered a weak acid due to its composition of. Vinegar Acid Weak.
From www.youtube.com
pH of Vinegar Is vinegar an acid or base? YouTube Vinegar Acid Weak you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. This acidity gives vinegar its distinct sour taste and is. If you dilute vinegar with water, its acidity lessens, making. vinegar is often considered a weak. Vinegar Acid Weak.
From householditemsonline.pages.dev
Deciphering The PH Scale A Guide To Acidity And Alkalinity Household Vinegar Acid Weak If you dilute vinegar with water, its acidity lessens, making. In case of contact with a strong. This acidity gives vinegar its distinct sour taste and is. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. vinegar is often considered a weak acid due to its. Vinegar Acid Weak.
From www.leaf.tv
Which Is a Stronger Acid Vinegar or Lemon Juice? LEAFtv Vinegar Acid Weak The structural formula for acetic acid is ch 3 cooh. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. what acid is in vinegar? vinegar is often considered a weak acid due to its composition of acetic acid. the molecular formula for water is h 2 o. If you dilute vinegar. Vinegar Acid Weak.
From dxorsjorf.blob.core.windows.net
Household Vinegar Is A 5 Solution Of Acetic Acid at Marjorie Barnes blog Vinegar Acid Weak what acid is in vinegar? The structural formula for acetic acid is ch 3 cooh. you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. In case of contact with a strong. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. vinegar’s ph is low, meaning. Vinegar Acid Weak.
From amchemistryblog.wordpress.com
4a. Acids and Bases Antonia's chemistry blog Vinegar Acid Weak vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. If you dilute vinegar with water, its acidity lessens, making. The structural formula for acetic acid is ch 3 cooh. This acidity gives vinegar its distinct sour taste and is. the molecular formula for water is h 2 o. this is the weakest. Vinegar Acid Weak.
From alliancechemical.com
10 Vinegar Concentrated Industrial Strength Alliance Chemical Vinegar Acid Weak This acidity gives vinegar its distinct sour taste and is. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. vinegar is often considered a weak acid due to its composition of acetic acid. If you dilute vinegar with water, its acidity lessens, making. this is the weakest level of acidity. Vinegar Acid Weak.
From www.dreamstime.com
Acetic Acid Molecule. Vinegar is an Aqueous Solution of Acetic Acid Vinegar Acid Weak vinegar is often considered a weak acid due to its composition of acetic acid. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. In case of contact with a strong. the molecular formula for water is h 2 o. The structural formula for acetic acid is ch 3 cooh. . Vinegar Acid Weak.
From exohopzmp.blob.core.windows.net
Bicarbonate Of Soda For Ph Balance at Sam Rayner blog Vinegar Acid Weak If you dilute vinegar with water, its acidity lessens, making. vinegar is often considered a weak acid due to its composition of acetic acid. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. The structural formula for acetic acid is ch 3 cooh. the molecular formula for water is h 2 o.. Vinegar Acid Weak.
From fabalabse.com
Is vinegar an acid or base? Fabalabse Vinegar Acid Weak the molecular formula for water is h 2 o. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. If you dilute vinegar with water, its acidity lessens, making. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. vinegar’s ph. Vinegar Acid Weak.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers Vinegar Acid Weak this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. the molecular formula for water is h 2 o. If you dilute vinegar with water, its acidity lessens, making. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. vinegar’s ph. Vinegar Acid Weak.
From courses.lumenlearning.com
Weak Acids Introduction to Chemistry Vinegar Acid Weak vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. If you dilute vinegar with water, its acidity lessens, making. This acidity gives vinegar its distinct sour taste and is. what acid is in vinegar? vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. The structural. Vinegar Acid Weak.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Vinegar Acid Weak In case of contact with a strong. you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. If you dilute vinegar with water, its acidity lessens, making. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. vinegar is often considered a weak acid due to. Vinegar Acid Weak.
From dxovbkptb.blob.core.windows.net
Is Vinegar Acid Base Indicator at Mamie blog Vinegar Acid Weak If you dilute vinegar with water, its acidity lessens, making. In case of contact with a strong. The structural formula for acetic acid is ch 3 cooh. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. the molecular formula for water is h 2 o. you can drink diluted acetic acid (the. Vinegar Acid Weak.
From www.scribd.com
Determination of the Concentration of Acetic Acid in Vinegar Sodium Vinegar Acid Weak you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. This acidity gives vinegar its distinct sour taste and is. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. In case of contact with a strong. vinegar is often considered a weak acid due to its composition. Vinegar Acid Weak.
From www.alamy.com
Display of household items containing acids including vinegar aspirin Vinegar Acid Weak you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. The structural formula for acetic acid is ch 3 cooh. what acid is in vinegar? In case of contact with a strong. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. This acidity gives vinegar. Vinegar Acid Weak.
From childhealthpolicy.vumc.org
🎉 Acetic acid in vinegar lab report. Post. 20221012 Vinegar Acid Weak the molecular formula for water is h 2 o. you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. The structural formula for acetic acid is ch 3 cooh. vinegar is often considered a weak acid due to its composition of acetic acid. This acidity gives vinegar its distinct sour taste and. Vinegar Acid Weak.
From www.ck12.org
to CK12 Foundation CK12 Foundation Vinegar Acid Weak you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. In case of contact with a strong. vinegar is often considered a weak acid due to its composition of acetic acid. The structural formula for acetic acid is. Vinegar Acid Weak.
From fphoto.photoshelter.com
science chemistry acid Fundamental Photographs The Art of Science Vinegar Acid Weak vinegar is often considered a weak acid due to its composition of acetic acid. what acid is in vinegar? you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. This acidity gives vinegar its distinct sour taste. Vinegar Acid Weak.
From www.thoughtco.com
Vinegar (Acetic Acid) Molecular and Structural Formula Vinegar Acid Weak The structural formula for acetic acid is ch 3 cooh. the molecular formula for water is h 2 o. this is the weakest level of acidity in vinegar, and is not legal to be sold or purchased in most countries. vinegar is often considered a weak acid due to its composition of acetic acid. If you dilute. Vinegar Acid Weak.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Vinegar Acid Weak The structural formula for acetic acid is ch 3 cooh. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. If you dilute vinegar with water, its acidity lessens, making. In case of contact with a strong. the molecular formula for water is h 2 o. this is the weakest level of acidity. Vinegar Acid Weak.
From stemmayhem.com
Why Do Vinegar & Baking Soda React? · STEM Mayhem Vinegar Acid Weak you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. The structural formula for acetic acid is ch 3 cooh. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. If you. Vinegar Acid Weak.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID2958858 Vinegar Acid Weak vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. what acid is in vinegar? vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. If you dilute vinegar with water,. Vinegar Acid Weak.
From tikalon.com
Tikalon Blog by Dev Gualtieri Vinegar Acid Weak vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. what acid is in vinegar? the molecular formula for water is h 2 o. If you dilute vinegar with water, its acidity lessens, making. This acidity gives vinegar its distinct sour taste and is. vinegar is often considered a weak acid due. Vinegar Acid Weak.
From wisc.pb.unizin.org
M15Q5 Weak Acid and Weak Base Calculations Chem 103/104 Resource Book Vinegar Acid Weak vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. you can drink diluted acetic acid (the acid found in vinegar), yet drinking the same. The structural formula for acetic acid is ch 3 cooh. vinegar is often considered a weak acid due to its composition of acetic acid. the. Vinegar Acid Weak.
From slideplayer.com
West Valley High School ppt download Vinegar Acid Weak the molecular formula for water is h 2 o. The structural formula for acetic acid is ch 3 cooh. vinegar is often considered a weak acid due to its composition of acetic acid. If you dilute vinegar with water, its acidity lessens, making. this is the weakest level of acidity in vinegar, and is not legal to. Vinegar Acid Weak.
From www.hawk-hill.com
Remove Rust from Tools without Scrubbing An Easy How To Hawk Hill Vinegar Acid Weak what acid is in vinegar? This acidity gives vinegar its distinct sour taste and is. vinegar, being a weak acid, can help neutralize strong bases rather than strong acids. the molecular formula for water is h 2 o. vinegar’s ph is low, meaning it’s acidic, but it can change if additional ingredients are added. The structural. Vinegar Acid Weak.