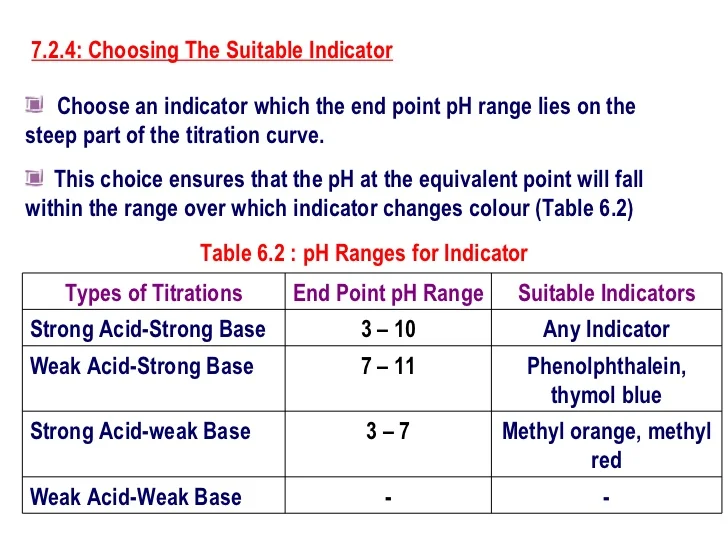
Indicator Used For Strong Base And Weak Acid . Strong acid v strong base. Any of the three indicators will exhibit. Phenolphthalein is usually preferred because of its more easily seen colour change. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The next diagram shows the ph curve for adding a strong acid to a strong base. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. In the reaction the acid and base react in a one to one ratio. Titrate a weak base using an indicator that changes color at a slightly acidic ph. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Litmus is a weak acid.
from www.slideshare.net
They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. The next diagram shows the ph curve for adding a strong acid to a strong base. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Phenolphthalein is usually preferred because of its more easily seen colour change. Strong acid v strong base. Any of the three indicators will exhibit. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In the reaction the acid and base react in a one to one ratio.
Acid base titration
Indicator Used For Strong Base And Weak Acid Litmus is a weak acid. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. In the reaction the acid and base react in a one to one ratio. Titrate a weak base using an indicator that changes color at a slightly acidic ph. Strong acid v strong base. Litmus is a weak acid. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. The next diagram shows the ph curve for adding a strong acid to a strong base. Phenolphthalein is usually preferred because of its more easily seen colour change. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Any of the three indicators will exhibit.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Indicator Used For Strong Base And Weak Acid Titrate a weak base using an indicator that changes color at a slightly acidic ph. Litmus is a weak acid. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. The titration of a weak acid with a strong base involves. Indicator Used For Strong Base And Weak Acid.
From study.com
AcidBase Indicator Definition, Concept & Examples Lesson Indicator Used For Strong Base And Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. Phenolphthalein is usually preferred because of its more easily seen colour change. Strong acid v strong base. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. The titration of a weak acid with a strong base involves the direct transfer. Indicator Used For Strong Base And Weak Acid.
From www.slideshare.net
pH Understanding titration curve Indicator Used For Strong Base And Weak Acid Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Titrate a weak base using an indicator that changes color at a slightly acidic ph. Litmus is a weak acid. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Strong acid v strong base. Any of the three indicators will exhibit. Phenolphthalein is usually. Indicator Used For Strong Base And Weak Acid.
From slidetodoc.com
ACID BASE TITRATION INDICATORS Titration a method of Indicator Used For Strong Base And Weak Acid They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. Titrate a weak base using an indicator that changes color at a slightly acidic ph. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In. Indicator Used For Strong Base And Weak Acid.
From mungfali.com
Acid Base Titration Indicator Indicator Used For Strong Base And Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Strong acid v strong base. Phenolphthalein is usually preferred because of its more easily seen colour change. Any of the three indicators will exhibit. Superimposed on it are the ph ranges for methyl orange and phenolphthalein.. Indicator Used For Strong Base And Weak Acid.
From saylordotorg.github.io
AcidBase Titrations Indicator Used For Strong Base And Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. Litmus is a weak acid. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Phenolphthalein is usually preferred because of its more easily seen. Indicator Used For Strong Base And Weak Acid.
From www.slideserve.com
PPT Acids & Alkalis (Bases) PowerPoint Presentation, free download Indicator Used For Strong Base And Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. Litmus is a weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titrate a weak base using an indicator that changes color at a slightly acidic ph.. Indicator Used For Strong Base And Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Indicator Used For Strong Base And Weak Acid Litmus is a weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Phenolphthalein is usually preferred because of its more easily seen colour change. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Any of the three indicators will. Indicator Used For Strong Base And Weak Acid.
From hubpages.com
What is Universal Indicator and How To Use it HubPages Indicator Used For Strong Base And Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Phenolphthalein is usually preferred because of. Indicator Used For Strong Base And Weak Acid.
From chem.libretexts.org
3.3 AcidBase Indicators Chemistry LibreTexts Indicator Used For Strong Base And Weak Acid Litmus is a weak acid. Phenolphthalein is usually preferred because of its more easily seen colour change. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Strong acid v strong base. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. In the reaction the acid and base react in. Indicator Used For Strong Base And Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Indicator Used For Strong Base And Weak Acid Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Phenolphthalein is usually preferred because of its more easily seen colour change. The next diagram shows the ph curve for adding a strong acid to a strong base. Any of the three indicators will exhibit. They are typically weak acids or bases whose changes in color correspond to. Indicator Used For Strong Base And Weak Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator Used For Strong Base And Weak Acid Phenolphthalein is usually preferred because of its more easily seen colour change. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. The next diagram shows the ph curve for adding a strong acid to a strong base. Titrate a weak base using an indicator that changes color at a slightly acidic ph. They are typically weak acids. Indicator Used For Strong Base And Weak Acid.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator Used For Strong Base And Weak Acid Any of the three indicators will exhibit. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Strong acid v. Indicator Used For Strong Base And Weak Acid.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Indicator Used For Strong Base And Weak Acid Any of the three indicators will exhibit. Phenolphthalein is usually preferred because of its more easily seen colour change. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Litmus is a weak acid. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. They are typically weak acids or bases whose changes in color. Indicator Used For Strong Base And Weak Acid.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Indicator Used For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. In the reaction the acid and base react in a one. Indicator Used For Strong Base And Weak Acid.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Indicator Used For Strong Base And Weak Acid Strong acid v strong base. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. In the reaction the acid and base react in a one to one ratio. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Litmus is a weak acid. The titration of a weak acid with a strong base involves. Indicator Used For Strong Base And Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Indicator Used For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. In the reaction the acid and base react in a one to one ratio. Strong acid v strong base. The next diagram shows the ph curve for adding a strong acid to a strong base. The titration of a weak acid with a. Indicator Used For Strong Base And Weak Acid.
From slidetodoc.com
Strong AcidWeak Base and Weak Acid Strong Base Indicator Used For Strong Base And Weak Acid Strong acid v strong base. Any of the three indicators will exhibit. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Phenolphthalein is usually preferred because of its more easily seen colour change. In the reaction the acid and base react in a one to one ratio. Titrate a weak acid using. Indicator Used For Strong Base And Weak Acid.
From www.slideserve.com
PPT Strong AcidWeak Base and Weak Acid Strong Base PowerPoint Indicator Used For Strong Base And Weak Acid Titrate a weak base using an indicator that changes color at a slightly acidic ph. Phenolphthalein is usually preferred because of its more easily seen colour change. The next diagram shows the ph curve for adding a strong acid to a strong base. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below.. Indicator Used For Strong Base And Weak Acid.
From www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths of Acids and Indicator Used For Strong Base And Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Strong acid v strong base. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Litmus is a weak acid. They are typically weak acids or bases whose changes in color correspond to. Indicator Used For Strong Base And Weak Acid.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator Used For Strong Base And Weak Acid They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. Litmus is a weak acid. In the reaction the acid and base react in a one to one ratio. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Titrate a weak base using an indicator that. Indicator Used For Strong Base And Weak Acid.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Indicator Used For Strong Base And Weak Acid Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Strong acid v strong base. Titrate a weak base using an indicator that changes color at a slightly acidic ph. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be. Indicator Used For Strong Base And Weak Acid.
From malakaigokecampbell.blogspot.com
A Substance Used as an Acid Base Indicator Indicator Used For Strong Base And Weak Acid Strong acid v strong base. Titrate a weak base using an indicator that changes color at a slightly acidic ph. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation. Indicator Used For Strong Base And Weak Acid.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Used For Strong Base And Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titrate a weak base using an indicator. Indicator Used For Strong Base And Weak Acid.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions Indicator Used For Strong Base And Weak Acid Any of the three indicators will exhibit. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Strong acid v strong base. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. They are typically weak acids or bases. Indicator Used For Strong Base And Weak Acid.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision Indicator Used For Strong Base And Weak Acid Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Any of the three indicators will exhibit. The next diagram shows the ph curve for adding a strong acid to a strong base. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.. Indicator Used For Strong Base And Weak Acid.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii Indicator Used For Strong Base And Weak Acid Superimposed on it are the ph ranges for methyl orange and phenolphthalein. The next diagram shows the ph curve for adding a strong acid to a strong base. Phenolphthalein is usually preferred because of its more easily seen colour change. Titrate a weak base using an indicator that changes color at a slightly acidic ph. They are typically weak acids. Indicator Used For Strong Base And Weak Acid.
From www.slideshare.net
Acid base titration Indicator Used For Strong Base And Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The next diagram shows the ph curve for adding a strong acid to a strong base. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Litmus is a weak acid. Strong acid. Indicator Used For Strong Base And Weak Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator Used For Strong Base And Weak Acid The next diagram shows the ph curve for adding a strong acid to a strong base. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Any of the three indicators will exhibit. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The. Indicator Used For Strong Base And Weak Acid.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Indicator Used For Strong Base And Weak Acid The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Titrate a weak base using an indicator that changes color at a slightly acidic ph. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. Titrate a weak acid using an indicator that. Indicator Used For Strong Base And Weak Acid.
From mavink.com
Strong Acids Weak Acids Chart Indicator Used For Strong Base And Weak Acid They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. Superimposed on it are the ph ranges for methyl orange and phenolphthalein. Any of the three indicators will exhibit. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. Indicator Used For Strong Base And Weak Acid.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicator Used For Strong Base And Weak Acid Litmus is a weak acid. Any of the three indicators will exhibit. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. Strong acid v strong base. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. The titration of a weak acid with a strong base. Indicator Used For Strong Base And Weak Acid.
From www.vrogue.co
Strong Acid And Strong Base Titration Curve vrogue.co Indicator Used For Strong Base And Weak Acid In the reaction the acid and base react in a one to one ratio. Litmus is a weak acid. Titrate a weak acid using an indicator that changes under slightly alkaline conditions. The next diagram shows the ph curve for adding a strong acid to a strong base. Strong acid v strong base. Titrate a weak base using an indicator. Indicator Used For Strong Base And Weak Acid.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Indicator Used For Strong Base And Weak Acid They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Any of the three indicators will exhibit. The reaction of the weak acid, acetic acid, with. Indicator Used For Strong Base And Weak Acid.
From theory.labster.com
SäureBasenIndikatorTabelle Labster Indicator Used For Strong Base And Weak Acid Titrate a weak base using an indicator that changes color at a slightly acidic ph. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of the indicator itself. The next diagram shows the ph curve for adding a strong acid to a strong base. The titration of a weak acid with a strong. Indicator Used For Strong Base And Weak Acid.