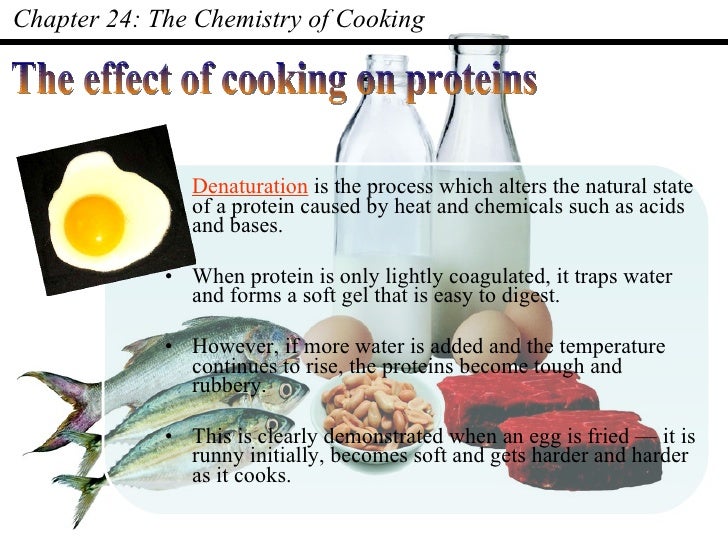
Egg Cooking Chemical Equation . Сао + н₂о = са (он)₂ + q. The longer an egg is cooked,. Water reacts with calcium oxide to form calcium hydroxide. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Explain your classification for each. Understanding these changes can help you understand the roles. During this reaction, so much heat is released that the liquid boils. Egg proteins change when you heat them, beat them, or mix them with other ingredients. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg.
from www.slideshare.net
The longer an egg is cooked,. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. During this reaction, so much heat is released that the liquid boils. Сао + н₂о = са (он)₂ + q. Water reacts with calcium oxide to form calcium hydroxide. Explain your classification for each. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Understanding these changes can help you understand the roles. Egg proteins change when you heat them, beat them, or mix them with other ingredients.
C24 the chemistry of cooking
Egg Cooking Chemical Equation Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. The longer an egg is cooked,. Explain your classification for each. Сао + н₂о = са (он)₂ + q. Egg proteins change when you heat them, beat them, or mix them with other ingredients. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. During this reaction, so much heat is released that the liquid boils. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. Water reacts with calcium oxide to form calcium hydroxide. Understanding these changes can help you understand the roles. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef.
From www.compoundchem.com
The Chemistry of Eggs & Egg Shells Compound Interest Egg Cooking Chemical Equation During this reaction, so much heat is released that the liquid boils. Understanding these changes can help you understand the roles. The longer an egg is cooked,. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Water reacts with calcium oxide to form calcium hydroxide. Сао + н₂о = са. Egg Cooking Chemical Equation.
From scienceline.org
A new equation can describe every egg Scienceline Egg Cooking Chemical Equation Egg proteins change when you heat them, beat them, or mix them with other ingredients. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Water reacts with calcium oxide to form calcium hydroxide. The longer an egg is cooked,. Explain your classification for each. The function. Egg Cooking Chemical Equation.
From www.expii.com
What Is a Chemical Reaction? — Overview & Examples Expii Egg Cooking Chemical Equation The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Water reacts with calcium oxide to form calcium hydroxide. Explain your classification for each. Сао + н₂о = са (он)₂ + q. Egg proteins change when you heat them, beat them, or mix them with other ingredients. During this reaction, so. Egg Cooking Chemical Equation.
From ar.inspiredpencil.com
Cooking An Egg Chemical Change Egg Cooking Chemical Equation Understanding these changes can help you understand the roles. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Explain your classification for each. Сао + н₂о = са (он)₂ + q. During this reaction, so much heat is released that the liquid boils. According to this. Egg Cooking Chemical Equation.
From loeprmyju.blob.core.windows.net
Worcestershire On Eggs at Allison King blog Egg Cooking Chemical Equation The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Explain your classification for each. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Water reacts with calcium oxide to form calcium hydroxide. Egg proteins change when. Egg Cooking Chemical Equation.
From www.youtube.com
Boiling an egg with chemistry! YouTube Egg Cooking Chemical Equation According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. Egg proteins change when you heat them, beat them, or mix them with other ingredients. Сао + н₂о = са (он)₂ + q. Eggs, one of the most ubiquitous ingredients in cooking,. Egg Cooking Chemical Equation.
From medium.com
The Universal Egg Equation. The perfect equation for the perfect… by Egg Cooking Chemical Equation Understanding these changes can help you understand the roles. Water reacts with calcium oxide to form calcium hydroxide. The longer an egg is cooked,. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Сао + н₂о = са (он)₂ + q. According to this formula, a. Egg Cooking Chemical Equation.
From www.youtube.com
Is Cooking An Egg A Chemical Change Or A Physical Change? YouTube Egg Cooking Chemical Equation The longer an egg is cooked,. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. Understanding these changes can help you understand the roles. Water reacts with calcium oxide to form calcium hydroxide. Egg proteins change when you heat them, beat. Egg Cooking Chemical Equation.
From tpsakihz.blogspot.com
Akih Tamaki Primary School Cooking egg! Egg Cooking Chemical Equation Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Egg proteins change when you heat them, beat them, or mix them with other ingredients. Сао + н₂о = са (он)₂ + q. The function of eggs eggs are a truly multifunctional ingredient and have many roles. Egg Cooking Chemical Equation.
From ar.inspiredpencil.com
Cooking An Egg Chemical Change Egg Cooking Chemical Equation Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Explain your classification for each. During this reaction, so much heat is released that the liquid boils. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Understanding. Egg Cooking Chemical Equation.
From www.slideserve.com
PPT Eggs PowerPoint Presentation, free download ID2982587 Egg Cooking Chemical Equation Egg proteins change when you heat them, beat them, or mix them with other ingredients. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Understanding these changes can help you understand the roles. During this reaction, so much heat is released that the liquid boils. Сао + н₂о = са. Egg Cooking Chemical Equation.
From www.mdpi.com
Foods Free FullText The Quality Characteristics Formation and Egg Cooking Chemical Equation Explain your classification for each. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Egg proteins change when you heat them, beat them, or mix them with other ingredients. Water reacts with calcium oxide to form calcium hydroxide. Understanding these changes can help you understand the roles. The longer an. Egg Cooking Chemical Equation.
From sciencenotes.org
Egg in Vinegar Experiment Make a Rubber Egg Egg Cooking Chemical Equation The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Explain your classification for each. Egg proteins change when you heat them, beat them, or mix them with other ingredients. Water reacts with calcium oxide to form calcium hydroxide. Understanding these changes can help you understand the roles. Eggs, one of. Egg Cooking Chemical Equation.
From www.youtube.com
Science progect. Cooking a egg. Chemical reaction YouTube Egg Cooking Chemical Equation The longer an egg is cooked,. During this reaction, so much heat is released that the liquid boils. Explain your classification for each. Understanding these changes can help you understand the roles. Сао + н₂о = са (он)₂ + q. Egg proteins change when you heat them, beat them, or mix them with other ingredients. The function of eggs eggs. Egg Cooking Chemical Equation.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID506027 Egg Cooking Chemical Equation Water reacts with calcium oxide to form calcium hydroxide. Egg proteins change when you heat them, beat them, or mix them with other ingredients. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. The longer an egg is cooked,. Understanding these. Egg Cooking Chemical Equation.
From www.haikudeck.com
Physical & Chemical Change by Matt Herberg Egg Cooking Chemical Equation Egg proteins change when you heat them, beat them, or mix them with other ingredients. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. Water reacts with calcium oxide to form calcium hydroxide. The longer an egg is cooked,. Eggs, one. Egg Cooking Chemical Equation.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Egg Cooking Chemical Equation During this reaction, so much heat is released that the liquid boils. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Water reacts with calcium oxide to form calcium hydroxide. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play. Egg Cooking Chemical Equation.
From www.slideshare.net
C24 the chemistry of cooking Egg Cooking Chemical Equation Explain your classification for each. The longer an egg is cooked,. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Сао + н₂о = са (он)₂ + q. Understanding these changes can help you understand the roles. Egg proteins change when you heat them, beat them, or mix them with. Egg Cooking Chemical Equation.
From cen.acs.org
Periodic graphics Dyeing Easter eggs Egg Cooking Chemical Equation Explain your classification for each. Understanding these changes can help you understand the roles. Сао + н₂о = са (он)₂ + q. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. Water reacts with calcium oxide to form calcium hydroxide. The. Egg Cooking Chemical Equation.
From www.youtube.com
Is cooking an egg a chemical change? YouTube Egg Cooking Chemical Equation The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. During this reaction, so much heat is released that the liquid boils. According to this formula, a medium. Egg Cooking Chemical Equation.
From www.pinterest.jp
Examples of Chemical changes 1)Rusting of Iron 2)burning of wood..3 Egg Cooking Chemical Equation During this reaction, so much heat is released that the liquid boils. Explain your classification for each. The longer an egg is cooked,. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Сао + н₂о = са (он)₂ + q. Water reacts with calcium oxide to form calcium hydroxide. Egg. Egg Cooking Chemical Equation.
From www.slideshare.net
C24 the chemistry of cooking Egg Cooking Chemical Equation The longer an egg is cooked,. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. During this reaction, so much heat is released that the liquid boils. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and. Egg Cooking Chemical Equation.
From www.pinterest.ph
Foodservices American Egg Board Egg facts, Eggs, Laying chickens Egg Cooking Chemical Equation According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. Сао + н₂о = са (он)₂ + q. Understanding these changes can help you understand the roles. Explain your classification for each. Water reacts with calcium oxide to form calcium hydroxide. Egg. Egg Cooking Chemical Equation.
From www.slideserve.com
PPT QUALITY OF EGG PowerPoint Presentation, free download ID6334061 Egg Cooking Chemical Equation Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Explain your classification for each. Сао + н₂о = са (он)₂ + q. Egg proteins change when you heat them, beat them, or mix them with other ingredients. Understanding these changes can help you understand the roles.. Egg Cooking Chemical Equation.
From melscience.com
Boiling an egg with chemistry! MEL Chemistry Egg Cooking Chemical Equation Сао + н₂о = са (он)₂ + q. Understanding these changes can help you understand the roles. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Explain your classification for each. During this reaction, so much heat is released that the liquid boils. Water reacts with calcium oxide to form. Egg Cooking Chemical Equation.
From www.slideserve.com
PPT Physical Change vs. Chemical Change PowerPoint Presentation, free Egg Cooking Chemical Equation Understanding these changes can help you understand the roles. Explain your classification for each. During this reaction, so much heat is released that the liquid boils. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg. Egg Cooking Chemical Equation.
From ar.inspiredpencil.com
Cooking An Egg Chemical Change Egg Cooking Chemical Equation The longer an egg is cooked,. Egg proteins change when you heat them, beat them, or mix them with other ingredients. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Explain your classification for each. Water reacts with calcium oxide to form calcium hydroxide. Сао +. Egg Cooking Chemical Equation.
From www.dreamstime.com
Structural Chemical Formula of Vitamin D with Fresh Eggs. High Vitamin Egg Cooking Chemical Equation Сао + н₂о = са (он)₂ + q. During this reaction, so much heat is released that the liquid boils. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Understanding these changes can help you understand the roles. The longer an egg is cooked,. Eggs, one of the most ubiquitous. Egg Cooking Chemical Equation.
From brainly.in
Chemical equation for frying an egg Brainly.in Egg Cooking Chemical Equation Water reacts with calcium oxide to form calcium hydroxide. Сао + н₂о = са (он)₂ + q. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. The function of eggs eggs are a truly multifunctional ingredient and have many roles to. Egg Cooking Chemical Equation.
From www.toppr.com
Write the balanced chemical equations for the following reactions and Egg Cooking Chemical Equation According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c) takes four and a half minutes to cook, but the same egg. Сао + н₂о = са (он)₂ + q. Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. Understanding. Egg Cooking Chemical Equation.
From ar.inspiredpencil.com
Cooking An Egg Chemical Change Egg Cooking Chemical Equation Understanding these changes can help you understand the roles. Сао + н₂о = са (он)₂ + q. Water reacts with calcium oxide to form calcium hydroxide. During this reaction, so much heat is released that the liquid boils. The longer an egg is cooked,. According to this formula, a medium egg (m~57 g) straight from the fridge (t egg =4°c). Egg Cooking Chemical Equation.
From www.dreamstime.com
Structural Chemical Formula of Vitamin D with Fresh Eggs. High Vitamin Egg Cooking Chemical Equation Eggs, one of the most ubiquitous ingredients in cooking, possess such a rich chemistry that they are absolute essentials to any serious chef. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Explain your classification for each. The longer an egg is cooked,. Egg proteins change when you heat them,. Egg Cooking Chemical Equation.
From animalia-life.club
Whipping Egg Whites Egg Cooking Chemical Equation The longer an egg is cooked,. Understanding these changes can help you understand the roles. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. During this reaction, so much heat is released that the liquid boils. Explain your classification for each. Water reacts with calcium oxide to form calcium hydroxide.. Egg Cooking Chemical Equation.
From ar.inspiredpencil.com
Cooking An Egg Chemical Change Egg Cooking Chemical Equation Сао + н₂о = са (он)₂ + q. Egg proteins change when you heat them, beat them, or mix them with other ingredients. During this reaction, so much heat is released that the liquid boils. The function of eggs eggs are a truly multifunctional ingredient and have many roles to play in the bakeshop. Water reacts with calcium oxide to. Egg Cooking Chemical Equation.
From www.chemistryviews.org
Egg White Chemistry ChemistryViews Egg Cooking Chemical Equation The longer an egg is cooked,. Water reacts with calcium oxide to form calcium hydroxide. During this reaction, so much heat is released that the liquid boils. Explain your classification for each. Egg proteins change when you heat them, beat them, or mix them with other ingredients. Understanding these changes can help you understand the roles. Сао + н₂о =. Egg Cooking Chemical Equation.