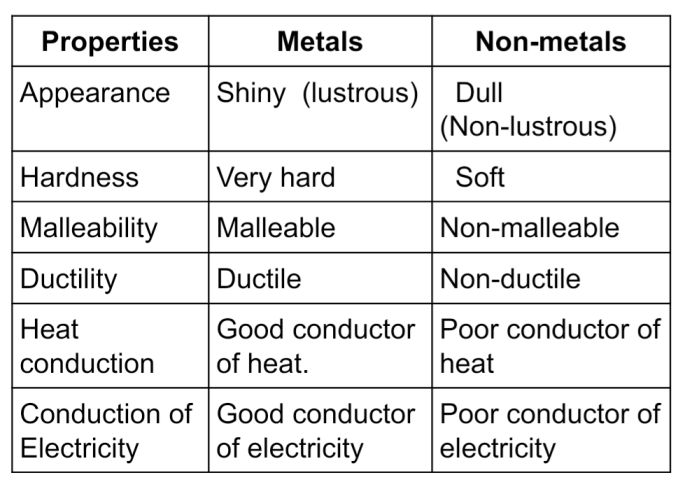
Metal Physical Characteristics . Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Thus metals are electropositive elements. List and explain the properties of metals. All the metals are good conductors of heat and electricity. Metals are solids at room temperature with the. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Remember that metals are on the left and bottom of the periodic table. Cooking utensils and irons are made up of metals as. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort.
from studymates91.blogspot.com
All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Remember that metals are on the left and bottom of the periodic table. List and explain the properties of metals. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Metals are solids at room temperature with the. Thus metals are electropositive elements. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Cooking utensils and irons are made up of metals as. All the metals are good conductors of heat and electricity.
CBSE Class 8 Science Revision Notes Chapter 4 Materials Metals and Non
Metal Physical Characteristics Metals are solids at room temperature with the. Thus metals are electropositive elements. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Remember that metals are on the left and bottom of the periodic table. List and explain the properties of metals. Metals are solids at room temperature with the. Cooking utensils and irons are made up of metals as. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. All the metals are good conductors of heat and electricity.
From www.iqsdirectory.com
Titanium Metal What Is It? How Is It Used? Properties Metal Physical Characteristics All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Remember that metals are on the left and bottom of the periodic table. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are solids at room temperature with the. Thus metals are electropositive elements. All the metals are good conductors. Metal Physical Characteristics.
From sciencenotes.org
Metals vs Nonmetals Metal Physical Characteristics Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. List and explain the properties of metals. All the metals are good conductors of heat and electricity. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Metals are lustrous, malleable,. Metal Physical Characteristics.
From studymates91.blogspot.com
CBSE Class 8 Science Revision Notes Chapter 4 Materials Metals and Non Metal Physical Characteristics Remember that metals are on the left and bottom of the periodic table. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. List and explain the properties of metals. All the metals are good conductors of heat and electricity. Cooking utensils and irons are made up of metals as. Metals are lustrous,. Metal Physical Characteristics.
From www.slideserve.com
PPT Transition Metals (Ligands) PowerPoint Presentation, free Metal Physical Characteristics Cooking utensils and irons are made up of metals as. All the metals are good conductors of heat and electricity. Remember that metals are on the left and bottom of the periodic table. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. List and explain the properties of metals. Metals. Metal Physical Characteristics.
From www.worksheetsplanet.com
Characteristics and Properties of Metals Metal Physical Characteristics List and explain the properties of metals. All the metals are good conductors of heat and electricity. Thus metals are electropositive elements. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Cooking utensils and irons are made up of metals as.. Metal Physical Characteristics.
From www.slideserve.com
PPT Transition Metals & Coordination Chemistry PowerPoint Metal Physical Characteristics List and explain the properties of metals. All the metals are good conductors of heat and electricity. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are solids at room temperature with the. Any component, no matter. Metal Physical Characteristics.
From www.differencebetween.net
Difference Between Metals, Metalloids, and Nonmetals Difference Between Metal Physical Characteristics Metals are solids at room temperature with the. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Cooking utensils and irons are made up of metals as. Thus metals are electropositive elements. All the metals are good conductors of heat and electricity. Any component, no matter how simple or. Metal Physical Characteristics.
From www.slideserve.com
PPT Physical Properties of Metals PowerPoint Presentation ID5519685 Metal Physical Characteristics Cooking utensils and irons are made up of metals as. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. List and explain the properties of metals. Thus metals are electropositive elements. All elements except hydrogen, which form positive ions. Metal Physical Characteristics.
From www.youtube.com
Physical and Chemical properties of the periodic table YouTube Metal Physical Characteristics Metals are solids at room temperature with the. Thus metals are electropositive elements. Remember that metals are on the left and bottom of the periodic table. Cooking utensils and irons are made up of metals as. List and explain the properties of metals. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals.. Metal Physical Characteristics.
From www.pinterest.com
7.6 Metals, Nonmetals, and Metalloids ppt video online download Metal Physical Characteristics Thus metals are electropositive elements. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Cooking utensils and irons are made up of metals as. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Remember that metals are on the left and. Metal Physical Characteristics.
From selftution.com
Physical and Chemical Properties of Metals » Selftution Metal Physical Characteristics Metals are solids at room temperature with the. Cooking utensils and irons are made up of metals as. Remember that metals are on the left and bottom of the periodic table. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Thus metals are electropositive elements. All elements except hydrogen,. Metal Physical Characteristics.
From www.slideserve.com
PPT METALS & NONMETALS PowerPoint Presentation, free download ID590868 Metal Physical Characteristics Thus metals are electropositive elements. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Metals are solids at room temperature with the. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Cooking utensils and irons are made up of metals as. Metals. Metal Physical Characteristics.
From brainly.in
State three differences between metallic elements and nonmetallic Metal Physical Characteristics Metals are lustrous, malleable, ductile, good conductors of heat and electricity. All the metals are good conductors of heat and electricity. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. List and explain the properties of metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as. Metal Physical Characteristics.
From studymind.co.uk
The Transition Metals (GCSE Chemistry) Study Mind Metal Physical Characteristics Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Metals are solids at room temperature with the. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Cooking utensils. Metal Physical Characteristics.
From www.slideshare.net
Metals, nonmetals, metalloids Metal Physical Characteristics Thus metals are electropositive elements. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Metals are solids at room temperature with the. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. List and explain the properties of metals. Cooking utensils and. Metal Physical Characteristics.
From selftution.com
Physical and Chemical Properties of Metals » Selftution Metal Physical Characteristics All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Thus metals are electropositive elements. List and explain the properties of metals. Metals are solids at room temperature with the. Remember that metals are on the left and bottom of the periodic table. Cooking utensils and irons are made up of metals as.. Metal Physical Characteristics.
From pixels.com
Physical Properties Of Metals And Alloys Photograph by Inna Bigun Metal Physical Characteristics Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. List and explain the properties of metals. All the metals are good conductors of heat and electricity. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Remember that metals are on the left and bottom of the periodic table.. Metal Physical Characteristics.
From brainly.in
State any five physical properties on the basis of which metals can be Metal Physical Characteristics Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Cooking utensils and irons are made up of metals as. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Thus metals are electropositive elements. List and explain the properties of metals. All elements except hydrogen, which form positive. Metal Physical Characteristics.
From sciencenotes.org
Metallic Character Trend on the Periodic Table Metal Physical Characteristics All the metals are good conductors of heat and electricity. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Cooking utensils and irons are made up of metals as. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Thus metals are. Metal Physical Characteristics.
From byjus.com
Properties of Metals And NonMetals Physical & Chemical Properties Metal Physical Characteristics Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Thus metals are electropositive elements. All elements except hydrogen, which. Metal Physical Characteristics.
From www.slideserve.com
PPT Materials for Inlays, Onlays, Crowns and Bridges PowerPoint Metal Physical Characteristics Cooking utensils and irons are made up of metals as. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. All the metals are good conductors of heat and electricity. Thus. Metal Physical Characteristics.
From www.alamy.com
Physical properties of metals and alloys, illustration Stock Photo Alamy Metal Physical Characteristics Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Remember that metals are on the left and bottom of the periodic table. Thus metals are electropositive elements. Cooking utensils and irons are made up of metals as. Any component, no matter how simple or complex, has to transmit or. Metal Physical Characteristics.
From animalia-life.club
Metallic Bond Examples List Metal Physical Characteristics Thus metals are electropositive elements. Remember that metals are on the left and bottom of the periodic table. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Metals are lustrous,. Metal Physical Characteristics.
From www.thoughtco.com
The Difference Between Metals and Nonmetals Metal Physical Characteristics List and explain the properties of metals. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Cooking utensils and irons are made up of metals as. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. All the metals are good conductors of. Metal Physical Characteristics.
From joivpoifp.blob.core.windows.net
Special Characteristics Of Iron at Melanie Price blog Metal Physical Characteristics List and explain the properties of metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. All the metals are good conductors of heat and electricity. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Any component, no matter how simple or complex, has to transmit or. Metal Physical Characteristics.
From www.slideserve.com
PPT CHAPTER 4 MATERIALS METALS AND NON METALS PowerPoint Metal Physical Characteristics Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Cooking utensils and irons are made up of metals as. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort.. Metal Physical Characteristics.
From www.haikudeck.com
IRON [Fe] by vannah.sanchez Metal Physical Characteristics All the metals are good conductors of heat and electricity. Thus metals are electropositive elements. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Cooking utensils and irons are made up of metals as. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Remember that metals are on the left. Metal Physical Characteristics.
From www.slideshare.net
Metals Physical Properties Metal Physical Characteristics Metals are solids at room temperature with the. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Cooking utensils and irons are made up of metals as. All the metals are good conductors of heat and electricity. Thus metals are electropositive. Metal Physical Characteristics.
From www.dreamstime.com
Properties of Metal and List of Physical Characteristics Outline Metal Physical Characteristics All the metals are good conductors of heat and electricity. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Metals are solids at room temperature with the. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. Cooking utensils and. Metal Physical Characteristics.
From www.slideserve.com
PPT Metals, Nonmetals, and Metalloids PowerPoint Presentation, free Metal Physical Characteristics Remember that metals are on the left and bottom of the periodic table. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Thus metals are electropositive elements. Metals are solids at room temperature with the. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. All elements except. Metal Physical Characteristics.
From kids.britannica.com
matter Students Britannica Kids Homework Help Metal Physical Characteristics Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Cooking utensils and irons are made up of metals as. Thus metals are electropositive elements. Any component, no matter how simple or complex, has to transmit or sustain a. Metal Physical Characteristics.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation, free Metal Physical Characteristics Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Cooking utensils and irons are made up of metals as. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort.. Metal Physical Characteristics.
From www.slideshare.net
Properties Of Metals Metal Physical Characteristics Thus metals are electropositive elements. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Remember that metals are on the left and bottom of the periodic table. Any component, no matter how simple or complex, has to transmit or sustain a mechanical load of some sort. All the metals. Metal Physical Characteristics.
From imeulia.blogspot.com
PRODUCT DESIGN Physical Properties of Metals Metal Physical Characteristics All the metals are good conductors of heat and electricity. Cooking utensils and irons are made up of metals as. List and explain the properties of metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Remember that metals are on the left and bottom of the periodic table.. Metal Physical Characteristics.
From www.youtube.com
PHYSICAL PROPERTIES OF METALS METALS AND NON METALSPART 2 YouTube Metal Physical Characteristics Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Metals are solids at room temperature with the. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Any component, no matter how simple or complex, has to transmit or sustain a mechanical. Metal Physical Characteristics.