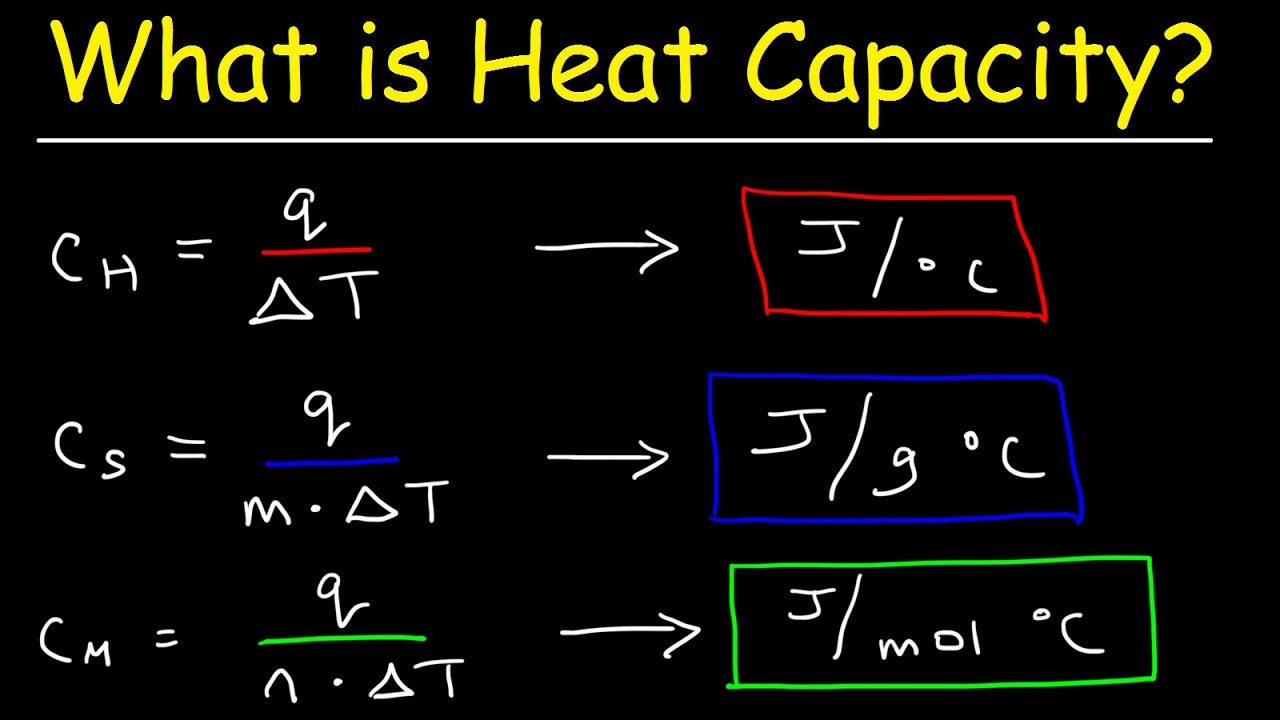
What Is Specific Heat Capacity Quizlet . Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. What is specific heat capacity? The specific heat capacity of a material is a. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It plays a crucial role in understanding. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. It describes how much heat must be added to a unit of mass of a given substance to raise its.
from www.youtube.com
Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It describes how much heat must be added to a unit of mass of a given substance to raise its. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. It plays a crucial role in understanding. What is specific heat capacity? The specific heat capacity of a material is a. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the.
What Is The Difference Between Specific Heat Capacity, Heat Capacity
What Is Specific Heat Capacity Quizlet It plays a crucial role in understanding. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. What is specific heat capacity? Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of a material is a. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It describes how much heat must be added to a unit of mass of a given substance to raise its.
From quizlet.com
Determination of specific heat capacity of a solid Diagram Quizlet What Is Specific Heat Capacity Quizlet Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. What is specific heat capacity? It plays a crucial role in understanding. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Study with quizlet. What Is Specific Heat Capacity Quizlet.
From studylib.net
Specific Heat Capacity Practice Problems What Is Specific Heat Capacity Quizlet What is specific heat capacity? Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree.. What Is Specific Heat Capacity Quizlet.
From quizlet.com
Specific Heat Capacity and Specific Latent Heat Flashcards Quizlet What Is Specific Heat Capacity Quizlet It plays a crucial role in understanding. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit. What Is Specific Heat Capacity Quizlet.
From worksheetlibrarysteins.z19.web.core.windows.net
Formula To Calculate Specific Heat What Is Specific Heat Capacity Quizlet Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. It plays a crucial role in understanding. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Its the amount of energy required to raise the temperature of. What Is Specific Heat Capacity Quizlet.
From www.worksheetsgo.com
Specific Heat Worksheets WorksheetsGO What Is Specific Heat Capacity Quizlet Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. What is specific heat capacity? Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. It describes how much heat must be added to a unit of mass of a. What Is Specific Heat Capacity Quizlet.
From slidetodoc.com
Specific Heat Capacity Objectives What is the difference What Is Specific Heat Capacity Quizlet Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. It describes how much heat must be added to a unit of mass of a given substance to raise its.. What Is Specific Heat Capacity Quizlet.
From quizlet.com
Specific Heat Capacity Required Practical, Physics, Year 9 GCSEs What Is Specific Heat Capacity Quizlet The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It describes how much heat must be added to a unit of mass of a given substance to raise its. The specific heat capacity of a material is a. What is specific heat capacity? Its the. What Is Specific Heat Capacity Quizlet.
From quizlet.com
The specific heat capacities for several substances are show Quizlet What Is Specific Heat Capacity Quizlet It describes how much heat must be added to a unit of mass of a given substance to raise its. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Specific heat is defined as the. What Is Specific Heat Capacity Quizlet.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Specific Heat Capacity Quizlet The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). What is specific heat capacity? Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It describes how much heat must. What Is Specific Heat Capacity Quizlet.
From edumentors.co.uk
GCSE Physics Particle model of matter Edumentors What Is Specific Heat Capacity Quizlet What is specific heat capacity? Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It describes how much heat must be added to a. What Is Specific Heat Capacity Quizlet.
From studylib.net
Heat Equation What Is Specific Heat Capacity Quizlet Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It plays a crucial role. What Is Specific Heat Capacity Quizlet.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Quizlet The specific heat capacity of a material is a. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It describes how much heat must be added to a unit of mass of a given substance to raise its. Its the amount of energy required to. What Is Specific Heat Capacity Quizlet.
From mungfali.com
Specific Heat Capacity Diagram What Is Specific Heat Capacity Quizlet Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of a material is a. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. It plays a crucial role in understanding. It describes how much heat must be added. What Is Specific Heat Capacity Quizlet.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Is Specific Heat Capacity Quizlet Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of. What Is Specific Heat Capacity Quizlet.
From www.studocu.com
Physics coursework What is Specific Heat Capacity? Specific heat What Is Specific Heat Capacity Quizlet It describes how much heat must be added to a unit of mass of a given substance to raise its. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the.. What Is Specific Heat Capacity Quizlet.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Specific Heat Capacity Quizlet It describes how much heat must be added to a unit of mass of a given substance to raise its. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. What is specific heat capacity? Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a. What Is Specific Heat Capacity Quizlet.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Is Specific Heat Capacity Quizlet The specific heat capacity of a material is a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. It plays a crucial role. What Is Specific Heat Capacity Quizlet.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Is Specific Heat Capacity Quizlet Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. What is specific heat capacity? Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. It plays a crucial role in understanding. It describes how much heat must be added to a unit of. What Is Specific Heat Capacity Quizlet.
From www.worksheetsplanet.com
What is Specific Heat What Is Specific Heat Capacity Quizlet The specific heat capacity of a material is a. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. It plays a crucial role in understanding. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. It describes how much. What Is Specific Heat Capacity Quizlet.
From www.youtube.com
3. 11P13.2 CV 2 Specific Heat Capacities of Gases and Mean Free Path What Is Specific Heat Capacity Quizlet Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It plays a crucial role in understanding. It describes how much heat must be added to a unit of mass of a given substance to raise its. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1. What Is Specific Heat Capacity Quizlet.
From quizlet.com
P2.3 Specific Heat Capacity (Practical) Diagram Quizlet What Is Specific Heat Capacity Quizlet Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). What is specific heat capacity? It plays a crucial role in understanding. Specific heat capacity is the amount of heat energy. What Is Specific Heat Capacity Quizlet.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Is Specific Heat Capacity Quizlet It describes how much heat must be added to a unit of mass of a given substance to raise its. Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise. What Is Specific Heat Capacity Quizlet.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Specific Heat Capacity Quizlet Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. It describes how much heat must be added to a unit of mass of. What Is Specific Heat Capacity Quizlet.
From www.vrogue.co
How To Work Out Specific Heat Capacity Perangkat Seko vrogue.co What Is Specific Heat Capacity Quizlet It describes how much heat must be added to a unit of mass of a given substance to raise its. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat is defined as the. What Is Specific Heat Capacity Quizlet.
From www.slideserve.com
PPT Heat (energy) Transfer PowerPoint Presentation, free download What Is Specific Heat Capacity Quizlet Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. The specific heat capacity of a material is a. What is specific heat capacity? The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It. What Is Specific Heat Capacity Quizlet.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Specific Heat Capacity Quizlet Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance. What Is Specific Heat Capacity Quizlet.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download What Is Specific Heat Capacity Quizlet Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the.. What Is Specific Heat Capacity Quizlet.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is Specific Heat Capacity Quizlet Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It describes how much heat must be. What Is Specific Heat Capacity Quizlet.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources What Is Specific Heat Capacity Quizlet Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. What is specific. What Is Specific Heat Capacity Quizlet.
From lessonmagicziegler.z21.web.core.windows.net
Worksheet Introduction To Specific Heat Capacities What Is Specific Heat Capacity Quizlet The specific heat capacity of a material is a. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. What is specific heat capacity? It describes how much heat must be added to a unit of. What Is Specific Heat Capacity Quizlet.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is Specific Heat Capacity Quizlet Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. The specific heat capacity of a material is the energy required to raise one kilogram (kg). What Is Specific Heat Capacity Quizlet.
From quizlet.com
Specific Heat Capacity REQUIRED PRACTICAL Diagram Quizlet What Is Specific Heat Capacity Quizlet Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). It plays a crucial role in understanding. The specific heat capacity of a material is a. Specific. What Is Specific Heat Capacity Quizlet.
From studymind.co.uk
Heat Capacity Study Mind What Is Specific Heat Capacity Quizlet Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Unlike the total heat capacity, the specific heat capacity is independent of mass or. What Is Specific Heat Capacity Quizlet.
From thechemistrynotes.com
Heat Capacity and Specific Heat What Is Specific Heat Capacity Quizlet Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat capacity of a material is a. What is specific heat capacity? Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the.. What Is Specific Heat Capacity Quizlet.
From www.transtutors.com
(Solved) 1. What Is The Average Specific Heat Capacity Of The Unknown What Is Specific Heat Capacity Quizlet Study with quizlet and memorise flashcards containing terms like what is specific heat capacity?, what are the factors involved if the. Its the amount of energy required to raise the temperature of 1 kilogram of the substance by 1 degree. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat is defined as. What Is Specific Heat Capacity Quizlet.