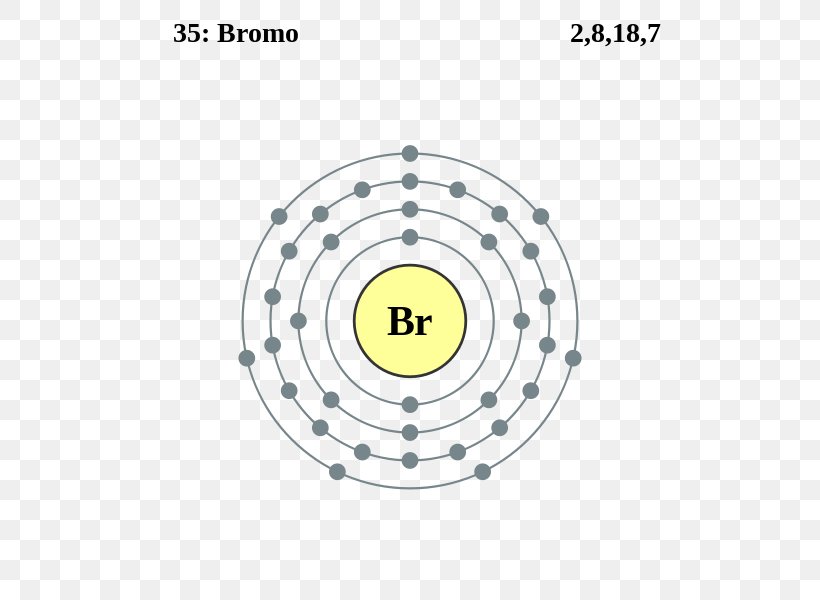
In Bromine Chloride Which Atom Is Slightly Negatively Charged . This table shows the most common charges for atoms of the chemical elements. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. You will find separate sections below covering. List of elements with their common. Ions can be positively charged or negatively charged. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. You can use this table to predict whether an. A lewis diagram is used to show how electrons are transferred to make ions and ionic.
from www.animalia-life.club
Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. You can use this table to predict whether an. List of elements with their common. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. You will find separate sections below covering. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Ions can be positively charged or negatively charged. This table shows the most common charges for atoms of the chemical elements. A lewis diagram is used to show how electrons are transferred to make ions and ionic.
Electron Configuration For Bromine
In Bromine Chloride Which Atom Is Slightly Negatively Charged This table shows the most common charges for atoms of the chemical elements. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Ions can be positively charged or negatively charged. A lewis diagram is used to show how electrons are transferred to make ions and ionic. This table shows the most common charges for atoms of the chemical elements. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. You will find separate sections below covering. List of elements with their common. You can use this table to predict whether an.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. When atoms gain electron/s, the negatively charged ion is formed, and when the. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration In Bromine Chloride Which Atom Is Slightly Negatively Charged This table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. You will find separate sections. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. List of elements with their common. Ions can be positively charged or negatively charged. This table shows the most common charges for atoms of the chemical elements. Atoms of the elements display a range of charges, but. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram In Bromine Chloride Which Atom Is Slightly Negatively Charged A lewis diagram is used to show how electrons are transferred to make ions and ionic. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine In Bromine Chloride Which Atom Is Slightly Negatively Charged A lewis diagram is used to show how electrons are transferred to make ions and ionic. List of elements with their common. You will find separate sections below covering. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Atoms of the elements display a range of. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) In Bromine Chloride Which Atom Is Slightly Negatively Charged When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. A lewis diagram is used to show how electrons are transferred to make ions and ionic. List of elements with their common. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From loeuqmdmn.blob.core.windows.net
Atomic Mass Of Bromine Class 9 at James Stevens blog In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. A lewis diagram is used to show how electrons are transferred to make ions and. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron In Bromine Chloride Which Atom Is Slightly Negatively Charged When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. This table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an. You will find separate sections below covering. Atoms of the elements display a range of charges,. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From ar.inspiredpencil.com
Atomic Structure Of Bromine In Bromine Chloride Which Atom Is Slightly Negatively Charged Ions can be positively charged or negatively charged. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When these atoms gain electrons,. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.coursehero.com
[Solved] The formal charge on the bromine atom in BrO 3 drawn with In Bromine Chloride Which Atom Is Slightly Negatively Charged This table shows the most common charges for atoms of the chemical elements. You will find separate sections below covering. A lewis diagram is used to show how electrons are transferred to make ions and ionic. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. List of. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. You can use this table to predict whether an. A lewis diagram is. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book In Bromine Chloride Which Atom Is Slightly Negatively Charged List of elements with their common. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. A lewis diagram is used to show how electrons are transferred to make ions and ionic. Atoms of the elements display a range of charges, but you can predict the most common. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram In Bromine Chloride Which Atom Is Slightly Negatively Charged You can use this table to predict whether an. Ions can be positively charged or negatively charged. List of elements with their common. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When these atoms gain electrons, they acquire a negative charge because they now. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.alamy.com
3d render of atom structure of bromine isolated over white background In Bromine Chloride Which Atom Is Slightly Negatively Charged When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. You will find separate sections below covering. This table shows the most common charges for atoms of the chemical elements. Ions can be positively charged or negatively charged. List of elements with their common. When these atoms gain. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image In Bromine Chloride Which Atom Is Slightly Negatively Charged A lewis diagram is used to show how electrons are transferred to make ions and ionic. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. This table shows the most common charges for atoms of the chemical elements. List of elements with their common. When. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From 123bike.biz
bromine electron configuration DrBeckmann In Bromine Chloride Which Atom Is Slightly Negatively Charged You can use this table to predict whether an. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. This table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br In Bromine Chloride Which Atom Is Slightly Negatively Charged A lewis diagram is used to show how electrons are transferred to make ions and ionic. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Atoms of the elements. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear In Bromine Chloride Which Atom Is Slightly Negatively Charged A lewis diagram is used to show how electrons are transferred to make ions and ionic. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From ar.inspiredpencil.com
Atomic Structure Of Bromine In Bromine Chloride Which Atom Is Slightly Negatively Charged This table shows the most common charges for atoms of the chemical elements. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. A lewis diagram is used to show how electrons are transferred to make ions and ionic. Atoms of the elements display a range of. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.istockphoto.com
Br Bromine Element Information Facts Properties Trends Uses And In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. This table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. A lewis diagram is used. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.coursehero.com
[Solved] Review the videos, and then identify the bromine (Br Br) atoms In Bromine Chloride Which Atom Is Slightly Negatively Charged This table shows the most common charges for atoms of the chemical elements. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Ions can be positively charged or negatively charged. You can use this table to predict whether an. Atoms that lose electrons acquire a. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.animalia-life.club
Electron Configuration For Bromine In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. You can use this table to predict whether an. Ions can be positively charged or negatively charged. A lewis diagram is used to show how electrons are transferred to make ions and ionic. When these atoms. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) In Bromine Chloride Which Atom Is Slightly Negatively Charged Ions can be positively charged or negatively charged. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. This table shows the most common charges for atoms of the chemical elements. Atoms that lose electrons acquire a positive charge as a result because they are left. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.istockphoto.com
Bromine Monochloride Molecular Structure Isolated On White Stock In Bromine Chloride Which Atom Is Slightly Negatively Charged This table shows the most common charges for atoms of the chemical elements. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. A lewis diagram is used to show how electrons are transferred to make ions and ionic. Atoms of the elements display a range of charges,. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. You will find separate sections below covering. This table shows the most common. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.animalia-life.club
Electron Configuration For Bromine In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. List of elements with their common. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. You can use this table to predict. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From material-properties.org
Bromine Periodic Table and Atomic Properties In Bromine Chloride Which Atom Is Slightly Negatively Charged When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. You will find separate sections below covering. A lewis diagram is used to show how electrons are transferred to make ions and ionic. List of elements with their common. This table shows the most common charges for atoms. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds In Bromine Chloride Which Atom Is Slightly Negatively Charged You can use this table to predict whether an. You will find separate sections below covering. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. List of elements with their common. When these atoms gain electrons, they acquire a negative charge because they now possess. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.animalia-life.club
Electron Configuration For Bromine In Bromine Chloride Which Atom Is Slightly Negatively Charged You will find separate sections below covering. This table shows the most common charges for atoms of the chemical elements. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements In Bromine Chloride Which Atom Is Slightly Negatively Charged A lewis diagram is used to show how electrons are transferred to make ions and ionic. Ions can be positively charged or negatively charged. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. You will find separate sections below covering. List of elements with their common. Atoms of the elements display. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms In Bromine Chloride Which Atom Is Slightly Negatively Charged You will find separate sections below covering. List of elements with their common. You can use this table to predict whether an. Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Ions can be positively charged or negatively charged. When atoms gain electron/s, the negatively. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From exoykphwz.blob.core.windows.net
Chlorine Electrons Number at Linwood Bell blog In Bromine Chloride Which Atom Is Slightly Negatively Charged When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. You will find separate sections below covering. This table shows the most common charges for atoms of the chemical elements.. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students In Bromine Chloride Which Atom Is Slightly Negatively Charged Atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. List of elements with their common. Ions can be positively charged or negatively. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.istockphoto.com
Infographic Of The Element Of Bromine Stock Illustration Download In Bromine Chloride Which Atom Is Slightly Negatively Charged List of elements with their common. A lewis diagram is used to show how electrons are transferred to make ions and ionic. Ions can be positively charged or negatively charged. This table shows the most common charges for atoms of the chemical elements. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer. In Bromine Chloride Which Atom Is Slightly Negatively Charged.
From www.animalia-life.club
Electron Configuration For Bromine In Bromine Chloride Which Atom Is Slightly Negatively Charged You will find separate sections below covering. You can use this table to predict whether an. List of elements with their common. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Ions can be positively charged or negatively charged. When atoms gain electron/s, the negatively charged ion is formed, and when. In Bromine Chloride Which Atom Is Slightly Negatively Charged.