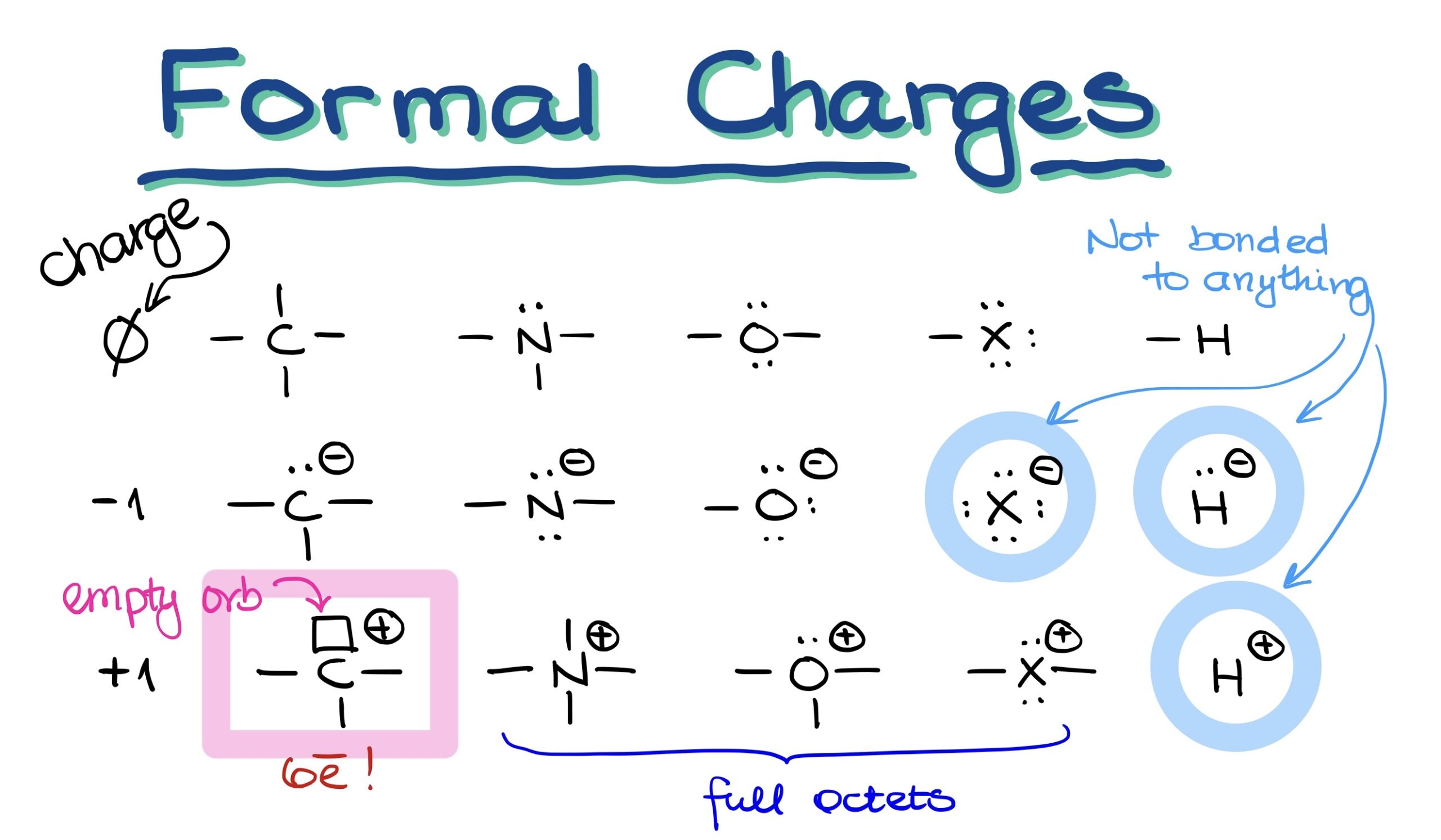
N2O Formal Charges . N 2 o is a good example of. Use the following formula to calculate the formal charges on atoms: N 2 o lewis structure formal charge. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. The formal charge is defined as the charge over a particular molecule assuming that all the atoms.
from www.organicchemistrytutor.com
Use the following formula to calculate the formal charges on atoms: N 2 o is a good example of. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o lewis structure formal charge.
Formal Charges — Organic Chemistry Tutor
N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o is a good example of. N 2 o lewis structure formal charge. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. Use the following formula to calculate the formal charges on atoms: The formal charge is defined as the charge over a particular molecule assuming that all the atoms.
From www.youtube.com
N2O Lewis Structure (Dinitrogen Oxide) YouTube N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. Use the following formula to calculate the formal charges on atoms: In order to calculate the formal charges for n2o. N2O Formal Charges.
From www.youtube.com
N2O Lewis Structure YouTube N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o is a good example of. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. In order to calculate the formal charges for n2o we'll use the. N2O Formal Charges.
From narodnatribuna.info
N2o Lewis Structure N2O Formal Charges In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. Use the following formula to calculate the formal charges on atoms: You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. The formal charge is defined as the charge over a. N2O Formal Charges.
From mavink.com
Formal Charge Lewis Structure N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. Use the following formula to calculate the formal charges on atoms: We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. You'll want to calculate the formal charges on each. N2O Formal Charges.
From www.numerade.com
SOLVED Assign formal charges to each atom in the resonance forms of N2O Formal Charges N 2 o is a good example of. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. Use the following formula to calculate the formal charges on atoms: In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. We can. N2O Formal Charges.
From www.dreamstime.com
Chemical Formulas of Nitrogen Oxide Nitric Oxide NO, Nitrogen Dioxide N2O Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o is a good example of. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. In order to calculate the formal charges for n2o we'll use the. N2O Formal Charges.
From aunitedkingdomfilm.com
Formal Charge N2o N2O Formal Charges In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o is a good example of. We can use the concept of formal charges to help us predict the. N2O Formal Charges.
From study.com
Calculating Formal Charge Definition & Formula Video & Lesson N2O Formal Charges In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. N 2 o lewis structure formal charge. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o is a good example of. You'll want to calculate the formal charges on each atom to make. N2O Formal Charges.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry N2O Formal Charges N 2 o is a good example of. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. Use the following formula to calculate the formal charges on atoms: N 2. N2O Formal Charges.
From www.toppr.com
Label formal charges and predict which of the following is the most N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for. N2O Formal Charges.
From www.chemistrylearner.com
Nitrite Ion (NO2) Formal Charge N2O Formal Charges N 2 o is a good example of. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. Use the following formula to calculate the formal charges on atoms: In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. N 2. N2O Formal Charges.
From ar.inspiredpencil.com
N2o Resonance Structures N2O Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. Use the following formula to calculate the formal charges on atoms: N 2 o. N2O Formal Charges.
From aunitedkingdomfilm.com
Formal Charge N2o N2O Formal Charges Use the following formula to calculate the formal charges on atoms: We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. You'll want to calculate the formal charges on each. N2O Formal Charges.
From www.slideserve.com
PPT Lewis Dot Diagrams and Formal Charges PowerPoint Presentation N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o lewis structure formal charge. N 2 o is a good example of. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. Use the following formula to. N2O Formal Charges.
From socratic.org
How to count formal charge in NO2? Socratic N2O Formal Charges We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o lewis. N2O Formal Charges.
From www.numerade.com
SOLVED Which statement rightly describes the Lewis structure of the N2O Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one. N2O Formal Charges.
From general.chemistrysteps.com
Formal Charges Chemistry Steps N2O Formal Charges N 2 o is a good example of. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. Use the following formula to calculate the formal charges on atoms: N 2 o lewis structure formal charge. The formal charge is defined as the charge over a particular. N2O Formal Charges.
From www.youtube.com
Chapter 8 Formal Charges of N2O Resonance Structures CHM 103 109 N2O Formal Charges We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o is a good example of. Use the following formula to calculate. N2O Formal Charges.
From aunitedkingdomfilm.com
Formal Charge N2o N2O Formal Charges Use the following formula to calculate the formal charges on atoms: You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o is a good example of. We can use the concept of formal charges to help us predict the most appropriate lewis structure when. N2O Formal Charges.
From www.numerade.com
SOLVED What is the correct Lewis structure for N2O, including the N2O Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. We can use the. N2O Formal Charges.
From byjus.com
Discuss resonance and formal charge in N_3^ and N_2O? N2O Formal Charges N 2 o lewis structure formal charge. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o is a good example of. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. You'll want to calculate the formal charges on each atom to make. N2O Formal Charges.
From clarkfvspears.blogspot.com
How to Calculate Formal Charge ClarkfvSpears N2O Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. The formal charge is defined as the charge over a particular molecule assuming that. N2O Formal Charges.
From www.youtube.com
Lewis structure of N2O and How To Draw The Lewis Structure Of N2O N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than. N2O Formal Charges.
From www.organicchemistrytutor.com
Formal Charges — Organic Chemistry Tutor N2O Formal Charges Use the following formula to calculate the formal charges on atoms: The formal charge is defined as the charge over a particular molecule assuming that all the atoms. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. In order to calculate the formal charges for n2o. N2O Formal Charges.
From www.numerade.com
SOLVED Question 19 5 points Save Answer Which statement rightly N2O Formal Charges We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. N 2 o is a good example of. Use the following formula to calculate the formal charges on atoms: N 2. N2O Formal Charges.
From www.bartleby.com
Answered The skeletal structure of N2Os is shown… bartleby N2O Formal Charges N 2 o lewis structure formal charge. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. Use the following formula to calculate the formal charges on atoms: In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. The formal charge. N2O Formal Charges.
From techiescientist.com
N2O Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram N2O Formal Charges We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. N 2 o lewis structure formal charge. N 2 o is a good example of. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. Use the following formula to calculate. N2O Formal Charges.
From www.chegg.com
Use formal charges for each of these four Lewis N2O Formal Charges In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. N 2 o is a good example of. You'll want to calculate the formal charges on each atom to make sure. N2O Formal Charges.
From www.youtube.com
N2O Molecular Geometry / Shape and Bond Angles YouTube N2O Formal Charges N 2 o lewis structure formal charge. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. Use the following formula to calculate the formal charges on atoms: You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2. N2O Formal Charges.
From www.numerade.com
SOLVED Given a possible Lewis structure of N2O below, what are the N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. Use the following formula. N2O Formal Charges.
From www.numerade.com
SOLVED A. The compound N2O has three (3) resonance forms (N in the N2O Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. Use the following formula to calculate the formal charges on atoms: N 2 o is a good example of. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. In. N2O Formal Charges.
From www.chegg.com
Solved Assign formal charges to each atom in the three N2O Formal Charges N 2 o is a good example of. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. We can use the concept of formal charges to help us predict. N2O Formal Charges.
From www.answersarena.com
[Solved] Determine the formal charge of nitrogen in the s N2O Formal Charges In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. N 2 o lewis structure formal charge. N 2 o is a good example of. The formal charge is defined as. N2O Formal Charges.
From byjus.com
48. Lewis dot structure of N2O. And formal charge on each atom. N2O Formal Charges In order to calculate the formal charges for n2o we'll use the equation:formal charge = [#. N 2 o is a good example of. We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. You'll want to calculate the formal charges on each atom to make sure. N2O Formal Charges.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry N2O Formal Charges We can use the concept of formal charges to help us predict the most appropriate lewis structure when more than one is reasonable. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. The formal charge is defined as the charge over a particular molecule assuming that. N2O Formal Charges.