Solution Dilution Worksheet . If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Use serial dilution to make a 2 mm solution from a 2 m stock solution. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. What is the concentration of zn 2+, in µg/ml, in your standard solution? If i dilute 250 ml. Next, you dilute 2.000 ml of this stock solution to 250.0 ml.
from www.docsity.com
If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. What is the concentration of zn 2+, in µg/ml, in your standard solution? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Use serial dilution to make a 2 mm solution from a 2 m stock solution. If i dilute 250 ml. Next, you dilute 2.000 ml of this stock solution to 250.0 ml. You are given a 1 m nacl solution and need a 10 mm nacl solution for.
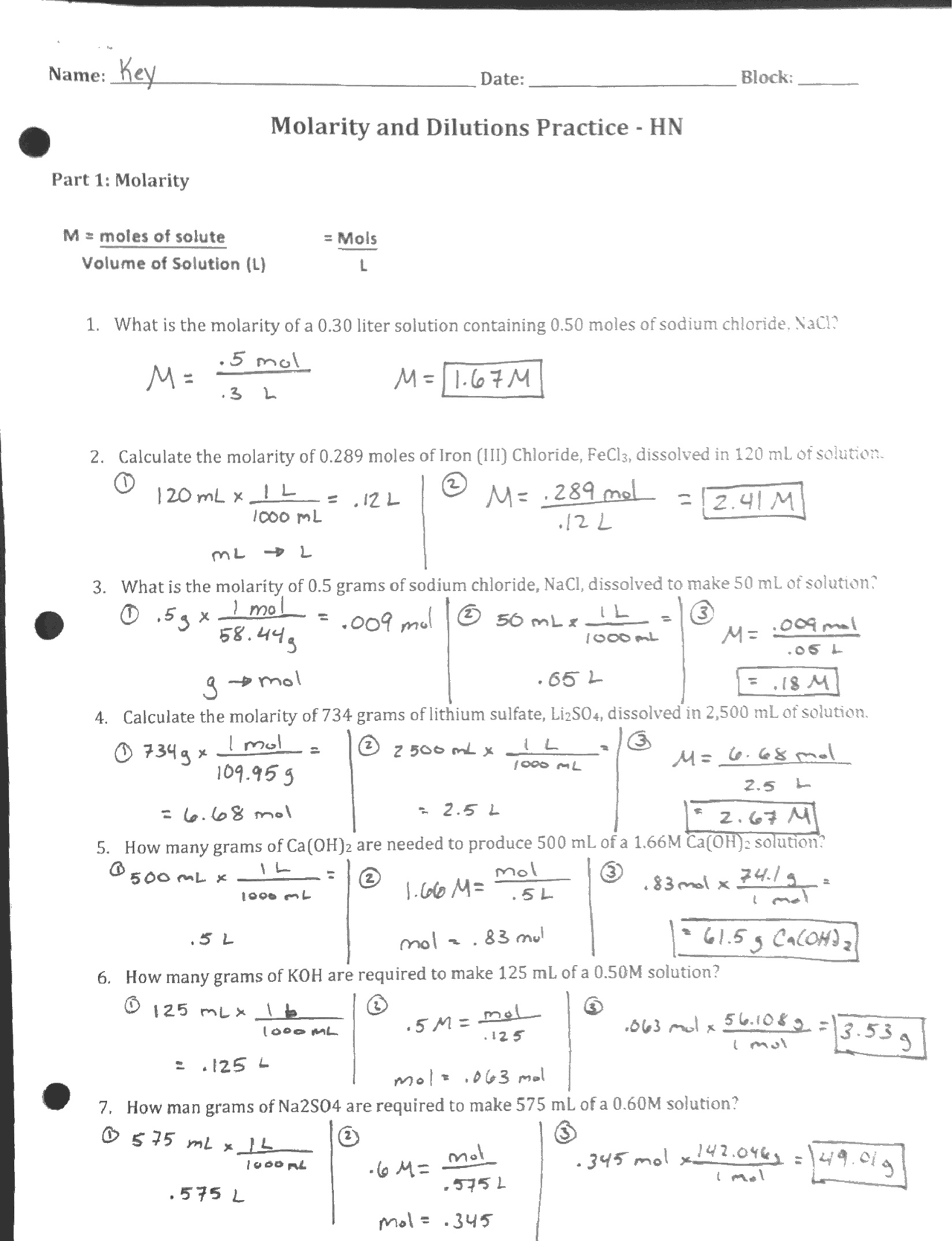
Molarity and Dilutions Practice Worksheet Key Docsity
Solution Dilution Worksheet Next, you dilute 2.000 ml of this stock solution to 250.0 ml. You are given a 1 m nacl solution and need a 10 mm nacl solution for. What is the concentration of zn 2+, in µg/ml, in your standard solution? Use serial dilution to make a 2 mm solution from a 2 m stock solution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? If i dilute 250 ml. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. Next, you dilute 2.000 ml of this stock solution to 250.0 ml.
From studycampusschreiber.z21.web.core.windows.net
Dilution Calculations Worksheet Solution Dilution Worksheet What is the concentration of zn 2+, in µg/ml, in your standard solution? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. If i dilute 250 ml. Next, you dilute 2.000 ml of this stock solution to 250.0 ml. You are given a. Solution Dilution Worksheet.
From materialzonetomlin.z21.web.core.windows.net
Dilution Problems Worksheets Solution Dilution Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. What is the concentration of zn 2+, in µg/ml, in your standard solution? If i dilute 250 ml. Next, you dilute 2.000 ml of this stock solution to 250.0 ml. If i have 340. Solution Dilution Worksheet.
From www.formsbank.com
Dilution Worksheet With Answers printable pdf download Solution Dilution Worksheet Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh. Solution Dilution Worksheet.
From studylib.net
Dilution Worksheet Solution Dilution Worksheet What is the concentration of zn 2+, in µg/ml, in your standard solution? You are given a 1 m nacl solution and need a 10 mm nacl solution for. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. If i dilute 250 ml.. Solution Dilution Worksheet.
From www.pinterest.co.uk
Introduction to Solutions Worksheet Set (Solutes, Solvents, and Solution Dilution Worksheet If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Next, you dilute 2.000 ml of this stock solution to 250.0 ml. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. Solution Dilution Worksheet.
From worksheets.ekocraft-appleleaf.com
Serial Dilution Worksheet Worksheets For Kindergarten Solution Dilution Worksheet If i dilute 250 ml. Next, you dilute 2.000 ml of this stock solution to 250.0 ml. What is the concentration of zn 2+, in µg/ml, in your standard solution? You are given a 1 m nacl solution and need a 10 mm nacl solution for. Use serial dilution to make a 2 mm solution from a 2 m stock. Solution Dilution Worksheet.
From worksheets.clipart-library.com
Dilution SelfStudy Worksheet Dilutions 1 Spring 2020 Dilutions Solution Dilution Worksheet You are given a 1 m nacl solution and need a 10 mm nacl solution for. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Use serial dilution to make a 2 mm solution from a 2 m stock solution. If i dilute 250. Solution Dilution Worksheet.
From www.chegg.com
Solved Name Period Date DILUTION WORKSHEET Direction Solve Solution Dilution Worksheet What is the concentration of zn 2+, in µg/ml, in your standard solution? If i dilute 250 ml. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Next, you dilute 2.000 ml of this stock solution to 250.0 ml. If i have 340. Solution Dilution Worksheet.
From www.chemistrylearner.com
Free Printable Molarity and Dilution Worksheets Solution Dilution Worksheet Use serial dilution to make a 2 mm solution from a 2 m stock solution. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. If i dilute 250 ml. What is the concentration of zn 2+, in µg/ml, in your standard solution? If i have 340 ml of a 0.5. Solution Dilution Worksheet.
From thekidsworksheet.com
Solutions And Dilutions Worksheet Thekidsworksheet Solution Dilution Worksheet Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. Next, you dilute 2.000 ml of this stock solution to 250.0 ml. What is the concentration of zn 2+, in µg/ml, in your standard solution? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh. Solution Dilution Worksheet.
From www.pinterest.com
Molarity and Dilutions Notes and Worksheet Set Teaching chemistry Solution Dilution Worksheet Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. What is the concentration of zn 2+, in µg/ml, in your standard solution? If i dilute 250 ml. You are given a 1 m nacl solution and need a 10 mm nacl solution for. Next, you dilute 2.000 ml of this. Solution Dilution Worksheet.
From www.scribd.com
Dilution Worksheet PDF Solution Dilution Worksheet Use serial dilution to make a 2 mm solution from a 2 m stock solution. What is the concentration of zn 2+, in µg/ml, in your standard solution? Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. You are given a 1 m nacl solution and need a 10 mm. Solution Dilution Worksheet.
From lessonanswerfullhartley.z22.web.core.windows.net
dilution worksheet chemistry Solution Dilution Worksheet You are given a 1 m nacl solution and need a 10 mm nacl solution for. Use serial dilution to make a 2 mm solution from a 2 m stock solution. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? What is the concentration. Solution Dilution Worksheet.
From kidsworksheetfun.com
Serial Dilutions Worksheet Answers Kidsworksheetfun Solution Dilution Worksheet Next, you dilute 2.000 ml of this stock solution to 250.0 ml. If i dilute 250 ml. Use serial dilution to make a 2 mm solution from a 2 m stock solution. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. 1) if i add 25 ml of water to. Solution Dilution Worksheet.
From worksheets.decoomo.com
20++ Dilutions Worksheet Answers Worksheets Decoomo Solution Dilution Worksheet What is the concentration of zn 2+, in µg/ml, in your standard solution? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml. Solution Dilution Worksheet.
From www.chegg.com
Solved Dilution diagrams can be very helpful in organizing Solution Dilution Worksheet Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. What is the concentration of zn 2+, in µg/ml, in your standard solution? If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Use serial dilution. Solution Dilution Worksheet.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Solution Dilution Worksheet What is the concentration of zn 2+, in µg/ml, in your standard solution? Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. You are given a 1 m nacl solution and need a 10 mm nacl solution for. If i dilute 250 ml. 1) if i add 25 ml of. Solution Dilution Worksheet.
From studyingworksheets.com
Saturated And Unsaturated Solutions Worksheet Studying Worksheets Solution Dilution Worksheet If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? If i dilute 250 ml. What is the concentration of zn 2+, in µg/ml, in your standard solution? You are given a 1 m nacl solution and need a 10 mm nacl solution for. Calculate. Solution Dilution Worksheet.
From www.chegg.com
Solved Dilution Worksheet 1. A stock solution of 1.00 M NaCl Solution Dilution Worksheet If i dilute 250 ml. What is the concentration of zn 2+, in µg/ml, in your standard solution? If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Next, you dilute 2.000 ml of this stock solution to 250.0 ml. Use serial dilution to make. Solution Dilution Worksheet.
From worksheets.clipart-library.com
Quiz & Worksheet How to Calculate Dilution of Solutions Solution Dilution Worksheet You are given a 1 m nacl solution and need a 10 mm nacl solution for. What is the concentration of zn 2+, in µg/ml, in your standard solution? Next, you dilute 2.000 ml of this stock solution to 250.0 ml. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will. Solution Dilution Worksheet.
From studylib.net
Solutions & Dilutions Worksheet Solution Dilution Worksheet What is the concentration of zn 2+, in µg/ml, in your standard solution? You are given a 1 m nacl solution and need a 10 mm nacl solution for. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Calculate the final concentration of a. Solution Dilution Worksheet.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Solution Dilution Worksheet If i dilute 250 ml. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? What is the concentration of zn 2+, in µg/ml, in your standard solution? You are given a 1 m nacl solution and need a 10 mm nacl solution for. 1). Solution Dilution Worksheet.
From study.com
Quiz & Worksheet How to Calculate Dilution of Solutions Solution Dilution Worksheet Use serial dilution to make a 2 mm solution from a 2 m stock solution. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? You are given a 1 m nacl solution and need a 10 mm nacl solution for. If i dilute 250. Solution Dilution Worksheet.
From worksheets.clipart-library.com
Dilution SelfStudy Worksheet Dilutions 1 Spring 2020 Dilutions Solution Dilution Worksheet Use serial dilution to make a 2 mm solution from a 2 m stock solution. If i dilute 250 ml. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity. Solution Dilution Worksheet.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Solution Dilution Worksheet If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? What is the concentration of zn 2+, in µg/ml, in your standard solution? Use serial dilution to make a 2 mm solution from a 2 m stock solution. Next, you dilute 2.000 ml of this. Solution Dilution Worksheet.
From www.chegg.com
Solved Dilution Worksheet 2 Show all work in the spaces Solution Dilution Worksheet What is the concentration of zn 2+, in µg/ml, in your standard solution? Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. You are given a 1 m nacl solution and need a 10 mm nacl solution for. If i dilute 250 ml. Use serial dilution to make a 2. Solution Dilution Worksheet.
From studylib.net
Dilutions Worksheet 11UChem Solution Dilution Worksheet What is the concentration of zn 2+, in µg/ml, in your standard solution? If i dilute 250 ml. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Use serial dilution to make a 2 mm solution from a 2 m stock solution. You are. Solution Dilution Worksheet.
From studylib.net
Concentration & Dilution Worksheet Solution Dilution Worksheet If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? You are given a 1 m nacl solution and need a 10 mm nacl solution for. What is the concentration of zn 2+, in µg/ml, in your standard solution? Next, you dilute 2.000 ml of. Solution Dilution Worksheet.
From www.chegg.com
Solved Dilution Worksheet 2 Show all work in the spaces Solution Dilution Worksheet If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? Next, you dilute 2.000 ml of this stock solution to 250.0 ml. If i dilute 250 ml. What is the concentration of zn 2+, in µg/ml, in your standard solution? You are given a 1. Solution Dilution Worksheet.
From www.chegg.com
Solved Dilution Worksheet 2 Show all work in the spaces Solution Dilution Worksheet If i dilute 250 ml. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. Use serial dilution to make a 2 mm solution from a 2 m stock solution. You are given a 1 m nacl solution and need a 10 mm nacl solution for. What is the concentration of. Solution Dilution Worksheet.
From www.chemistryworksheet.com
Dilution Calculation Worksheet With Answers Printable Pdf Download Solution Dilution Worksheet Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. Next, you dilute 2.000 ml of this stock solution to 250.0 ml. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? 1) if i add. Solution Dilution Worksheet.
From studylib.net
Dilutions Worksheet Solution Dilution Worksheet Next, you dilute 2.000 ml of this stock solution to 250.0 ml. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in. If i dilute. Solution Dilution Worksheet.
From db-excel.com
Quiz Worksheet How To Calculate Dilution Of Solutions — Solution Dilution Worksheet If i dilute 250 ml. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. What is the concentration of zn 2+, in µg/ml, in your standard solution? You are given a 1 m nacl solution and need a 10 mm nacl solution for.. Solution Dilution Worksheet.
From www.liveworksheets.com
Preparing Standard Solution Through Dilution method worksheet Live Solution Dilution Worksheet You are given a 1 m nacl solution and need a 10 mm nacl solution for. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide. Solution Dilution Worksheet.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Solution Dilution Worksheet Use serial dilution to make a 2 mm solution from a 2 m stock solution. If i have 340 ml of a 0.5 m nabr solution, what will the concentration be if i add 560 ml more water to it? You are given a 1 m nacl solution and need a 10 mm nacl solution for. Next, you dilute 2.000. Solution Dilution Worksheet.