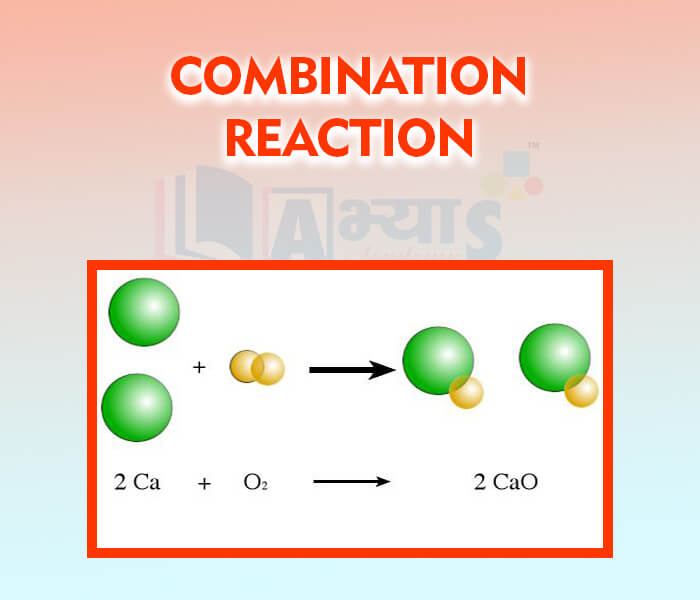
Combination Reaction Definition . A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A combination reaction is also known as a synthesis reaction. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is a reaction where two reactants are combined into one product. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. It is also called a synthesis. A combination reaction is a reaction in which two or more substances combine to form a single new substance.
from ar.inspiredpencil.com
A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. A combination reaction is also known as a synthesis reaction. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. It is also called a synthesis. A combination reaction is a reaction in which two or more substances combine to form a single new substance. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction is a reaction where two reactants are combined into one product. Learn how to describe and identify different types of combination reactions using chemical equations.
Combination Reaction
Combination Reaction Definition A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. A combination reaction is a reaction where two reactants are combined into one product. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A combination reaction is a reaction in which two or more substances combine to form a single new substance. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction is also known as a synthesis reaction. It is also called a synthesis. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. Learn how to describe and identify different types of combination reactions using chemical equations.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Combination Reaction Definition Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is a reaction where two reactants are combined into one product. A combination reaction is also known as a synthesis reaction. It. Combination Reaction Definition.
From buchanonlibrary.blogspot.com
Combination Reaction Worksheet 1 Buchanon Library Combination Reaction Definition A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. It is also called a synthesis. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction is also known as a synthesis reaction. A combination reaction,. Combination Reaction Definition.
From www.youtube.com
combination reaction // class 10th // ncert YouTube Combination Reaction Definition A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. It is also called a synthesis. A combination reaction is also known as a synthesis reaction. Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is a reaction in which two. Combination Reaction Definition.
From www.youtube.com
Combination reaction & Synthesis reaction // Definition with Reactions Combination Reaction Definition Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a. Combination Reaction Definition.
From www.youtube.com
Types of Reaction Part 1 Combination reaction YouTube Combination Reaction Definition Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. It is also called a synthesis. A combination reaction is also known as a synthesis reaction. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A. Combination Reaction Definition.
From www.youtube.com
Combination reaction 05 YouTube Combination Reaction Definition A combination reaction is a reaction where two reactants are combined into one product. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is also known as a synthesis reaction. A. Combination Reaction Definition.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Combination Reaction Definition It is also called a synthesis. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction is also known as a synthesis reaction. Learn. Combination Reaction Definition.
From www.vrogue.co
Single Replacement Reactions Definition Examples Expi vrogue.co Combination Reaction Definition A combination reaction is a reaction where two reactants are combined into one product. It is also called a synthesis. A combination reaction is a reaction in which two or more substances combine to form a single new substance. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex. Combination Reaction Definition.
From gbu-taganskij.ru
What Is A Synthesis Reaction? Definition And Examples, 43 OFF Combination Reaction Definition It is also called a synthesis. Learn how to describe and identify different types of combination reactions using chemical equations. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction is a reaction in which two or more substances combine to form a single new substance.. Combination Reaction Definition.
From www.youtube.com
Chemical Reaction and Equation Reaction) class 10th Combination Reaction Definition Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction is a reaction where two reactants are combined into one product. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Learn how to describe and identify. Combination Reaction Definition.
From studyqueries.com
What Is Combination Reaction? Definition, Examples Combination Reaction Definition A combination reaction is also known as a synthesis reaction. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. It is also called a synthesis. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A synthesis. Combination Reaction Definition.
From gbu-taganskij.ru
What Is A Synthesis Reaction? Definition And Examples, 43 OFF Combination Reaction Definition A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. A combination reaction is a reaction in which two or more substances combine to form a single new substance. It is also called a synthesis. A combination reaction is also known as a synthesis reaction. Learn how to describe and. Combination Reaction Definition.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Combination Reaction Definition Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction is also known as a synthesis reaction. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A combination reaction, also known as a. Combination Reaction Definition.
From www.tessshebaylo.com
Single Replacement Reaction Balanced Chemical Equation Tessshebaylo Combination Reaction Definition Learn how to describe and identify different types of combination reactions using chemical equations. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A combination reaction. Combination Reaction Definition.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Combination Reaction Definition Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is a reaction where two reactants are combined into one product. A combination reaction is also known as a synthesis reaction. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A. Combination Reaction Definition.
From ar.inspiredpencil.com
Combination Reaction Combination Reaction Definition A combination reaction is a reaction where two reactants are combined into one product. It is also called a synthesis. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single. Combination Reaction Definition.
From sciencenotes.org
What Is a Chemical Reaction? Definition and Examples Combination Reaction Definition A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. It is also called a synthesis. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. Learn how to describe and identify different types of combination reactions. Combination Reaction Definition.
From www.slideserve.com
PPT Writing Chemical Reactions PowerPoint Presentation, free download Combination Reaction Definition A combination reaction is a reaction where two reactants are combined into one product. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is also known as a synthesis reaction. A. Combination Reaction Definition.
From www.thoughtco.com
Types of Chemical Reactions (With Examples) Combination Reaction Definition A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. It is also. Combination Reaction Definition.
From brainly.in
define combination reaction Brainly.in Combination Reaction Definition A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. Learn how to describe and identify different types of combination reactions using chemical equations. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A combination reaction is. Combination Reaction Definition.
From www.aakash.ac.in
reaction Definition, Classification, Uses and Importance Combination Reaction Definition It is also called a synthesis. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. Learn how to describe and identify different types of combination reactions using chemical equations. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more. Combination Reaction Definition.
From armsingle10.pythonanywhere.com
Nice Examples For Chemical Combination Vector Algebra Class 12 Notes Combination Reaction Definition A combination reaction is a reaction where two reactants are combined into one product. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. It is also called a synthesis. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single. Combination Reaction Definition.
From www.teachoo.com
Combination Reaction Definition with 4 Examples Class 10 Chemistry Combination Reaction Definition A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with. Combination Reaction Definition.
From sciencenotes.org
Double Replacement Reaction Definition and Examples Combination Reaction Definition Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is also known as a synthesis reaction. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. A combination reaction is a reaction in which two or more substances combine to form. Combination Reaction Definition.
From sciencenotes.org
What Is a Synthesis Reaction? Definition and Examples Combination Reaction Definition Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is. Combination Reaction Definition.
From www.teachoo.com
NCERT Exemplar (MCQ) Which of the following are combination reaction Combination Reaction Definition Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and compare them with decomposition reactions. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. Learn how to describe and identify different types of combination reactions using chemical equations. A synthesis reaction is. Combination Reaction Definition.
From ar.inspiredpencil.com
Combination Reaction Combination Reaction Definition Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction is also known as a synthesis reaction. A combination reaction is a reaction where two reactants are combined into one product. It is also called a synthesis. Learn how to identify a synthesis reaction, see examples of different types of synthesis reactions, and. Combination Reaction Definition.
From www.slideserve.com
PPT Chapter 5 Chemical Reactions PowerPoint Presentation, free Combination Reaction Definition A combination reaction is a reaction where two reactants are combined into one product. A combination reaction is also known as a synthesis reaction. Learn how to describe and identify different types of combination reactions using chemical equations. It is also called a synthesis. A combination reaction is a reaction in which two or more substances combine to form a. Combination Reaction Definition.
From byjus.com
Types of Chemical Reactions Detailed Explanation With Example & Videos Combination Reaction Definition It is also called a synthesis. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. A combination reaction is a reaction in which two or more substances combine. Combination Reaction Definition.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Combination Reaction Definition A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. A combination reaction is a reaction in which two or more substances combine to form a single new. Combination Reaction Definition.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Combination Reaction Definition A combination reaction is a reaction in which two or more substances combine to form a single new substance. Learn how to describe and identify different types of combination reactions using chemical equations. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. A synthesis reaction is a chemical. Combination Reaction Definition.
From study.com
How to Identify a Combination Reaction Chemistry Combination Reaction Definition A combination reaction is a reaction in which two or more substances combine to form a single new substance. A combination reaction is a reaction where two reactants are combined into one product. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a single product. It is also called a synthesis.. Combination Reaction Definition.
From prabhakarpk.blogspot.com
Chemical Reaction and its types Combination Reaction Definition A combination reaction is a reaction where two reactants are combined into one product. A combination reaction is a reaction in which two or more substances combine to form a single new substance. A combination reaction is a type of chemical reaction where two or more reactants combine to form a single product. A synthesis reaction is a chemical reaction. Combination Reaction Definition.
From studyele1metw.z21.web.core.windows.net
Types Of Chemical Reaction Worksheets Combination Reaction Definition A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A combination reaction is a reaction in which two or more substances combine to form a single new substance. A combination reaction, also known as a synthesis reaction, occurs when two or more reactants combine to form a. Combination Reaction Definition.
From www.filscihub.com
[CHEMISTRY MODULE] Combination Reactions — Filipino Science Hub Combination Reaction Definition A combination reaction is a reaction where two reactants are combined into one product. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. It is also called a synthesis. A combination reaction is a reaction in which two or more substances combine to form a single new. Combination Reaction Definition.