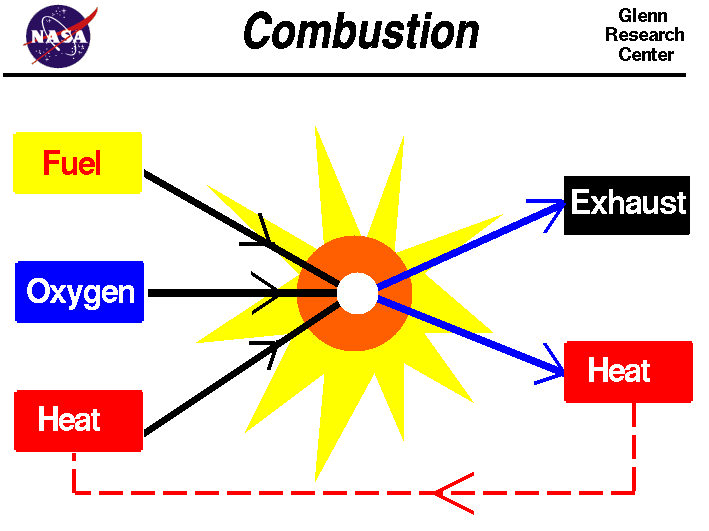
Fuel In Chemical Reaction . The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen.
from www.grc.nasa.gov
Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
Combustion
Fuel In Chemical Reaction Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen.
From sciencenotes.org
Combustion Reaction Definition and Examples Fuel In Chemical Reaction A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). The products of the combustion of hydrocarbons are always carbon dioxide and. Many. Fuel In Chemical Reaction.
From www.youtube.com
Evidence of Chemical Change Production of a Gas YouTube Fuel In Chemical Reaction The products of the combustion of hydrocarbons are always carbon dioxide and. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is an exothermic chemical reaction between substances,. Fuel In Chemical Reaction.
From www.vectorstock.com
Molten carbonate fuel cells process Royalty Free Vector Fuel In Chemical Reaction Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas. Fuel In Chemical Reaction.
From chemistry-europe.onlinelibrary.wiley.com
Thermochemical Conversion of Plastic Waste into Fuels, Chemicals, and Fuel In Chemical Reaction The products of the combustion of hydrocarbons are always carbon dioxide and. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is an exothermic chemical reaction. Fuel In Chemical Reaction.
From www.shalom-education.com
Fuels and Combustion Shalom Education Fuel In Chemical Reaction A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The products of the combustion of hydrocarbons are always carbon dioxide. Fuel In Chemical Reaction.
From www.grc.nasa.gov
Combustion Fuel In Chemical Reaction Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen. Fuel In Chemical Reaction.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages Fuel In Chemical Reaction A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy,. Fuel In Chemical Reaction.
From www.syngaschem.com
Synthesis Gas Chemistry and Synthetic Fuels Syngaschem BV Fuel In Chemical Reaction Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is a reaction in which a substance reacts with oxygen gas,. Fuel In Chemical Reaction.
From www.youtube.com
Chem143 Gas Producing Chemical Reactions YouTube Fuel In Chemical Reaction The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of. Fuel In Chemical Reaction.
From www.chemicals.co.uk
Examples of Combustion Reactions in Chemistry The Chemistry Blog Fuel In Chemical Reaction The products of the combustion of hydrocarbons are always carbon dioxide and. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). A combustion reaction is one in which. Fuel In Chemical Reaction.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Fuel In Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the. Fuel In Chemical Reaction.
From www.teachoo.com
Case Based MCQ Chemistry in Automobiles For an internal combustion Fuel In Chemical Reaction The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. Many combustion reactions. Fuel In Chemical Reaction.
From www.chemistrylearner.com
Gas Evolution Reaction Definition and Examples Fuel In Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation. Fuel In Chemical Reaction.
From sciencetallis.weebly.com
7. Organic Chemistry THOMAS TALLIS SCIENCE Fuel In Chemical Reaction A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The products of the combustion of hydrocarbons are always carbon dioxide. Fuel In Chemical Reaction.
From www.youtube.com
Video Demonstration Chemical Reactions Involving Gas Formation Part 2 Fuel In Chemical Reaction The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Many combustion reactions. Fuel In Chemical Reaction.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Fuel In Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and. Fuel In Chemical Reaction.
From www.youtube.com
Hydrogen & Fuel Cells Reactions Chemistry FuseSchool YouTube Fuel In Chemical Reaction A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The products of the combustion of hydrocarbons are always carbon dioxide and. Many combustion. Fuel In Chemical Reaction.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Fuel In Chemical Reaction Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and. Fuel In Chemical Reaction.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Fuel In Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the. Fuel In Chemical Reaction.
From www.shutterstock.com
Gas Reaction Equation Gas Evolution Reaction Stock Vector (Royalty Free Fuel In Chemical Reaction Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas. Fuel In Chemical Reaction.
From www.compoundchem.com
The Chemistry of Petrol & The Tetraethyl Lead Story Compound Interest Fuel In Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the. Fuel In Chemical Reaction.
From www.dreamstime.com
Hydrogen Fuel Cell. Chemical Reaction Producing Electricity. H2 Energy Fuel In Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied. Fuel In Chemical Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Fuel In Chemical Reaction A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The products of the combustion of hydrocarbons are always carbon dioxide. Fuel In Chemical Reaction.
From www.alamy.com
Illustration depicting how a fuel cell converts the chemical energy Fuel In Chemical Reaction Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat. Fuel In Chemical Reaction.
From revisechemistry.uk
Carbon Compounds as Fuels and Feedstock AQA C7 revisechemistry.uk Fuel In Chemical Reaction Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is an exothermic chemical reaction. Fuel In Chemical Reaction.
From www.elephango.com
Describing Chemical Reactions Educational Resources K12 Learning Fuel In Chemical Reaction A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation. Fuel In Chemical Reaction.
From www.youtube.com
Balancing combustion reaction equation Gasoline Chemistry YouTube Fuel In Chemical Reaction Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of. Fuel In Chemical Reaction.
From www.researchgate.net
Scheme of a solid oxide fuel cell. Download Scientific Diagram Fuel In Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied. Fuel In Chemical Reaction.
From www.youtube.com
Chemistry Lesson Gas Evolution Reactions YouTube Fuel In Chemical Reaction A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The products of the combustion of hydrocarbons are always carbon dioxide and. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is an exothermic chemical reaction between. Fuel In Chemical Reaction.
From large.stanford.edu
Hydrogen Fuel Cells Fuel In Chemical Reaction Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light. Fuel In Chemical Reaction.
From www.researchgate.net
Chemical reaction in proton exchange membrane fuel cell (PEMFC Fuel In Chemical Reaction A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is one in which a substance reacts with oxygen. Fuel In Chemical Reaction.
From www.dreamstime.com
Fuel Combustion Process. Different Types of Fuel. Vector Illustration Fuel In Chemical Reaction The products of the combustion of hydrocarbons are always carbon dioxide and. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.. Fuel In Chemical Reaction.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel In Chemical Reaction A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and. Fuel In Chemical Reaction.
From rolfcandy.weebly.com
Cathode reaction in hydrogen fuel cell rolfcandy Fuel In Chemical Reaction The products of the combustion of hydrocarbons are always carbon dioxide and. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including oxygen. Fuel In Chemical Reaction.
From www.youtube.com
Balancing Combustion Reactions YouTube Fuel In Chemical Reaction A combustion reaction is one in which a substance reacts with oxygen gas, releasing energy in the form of heat and light. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas. Fuel In Chemical Reaction.