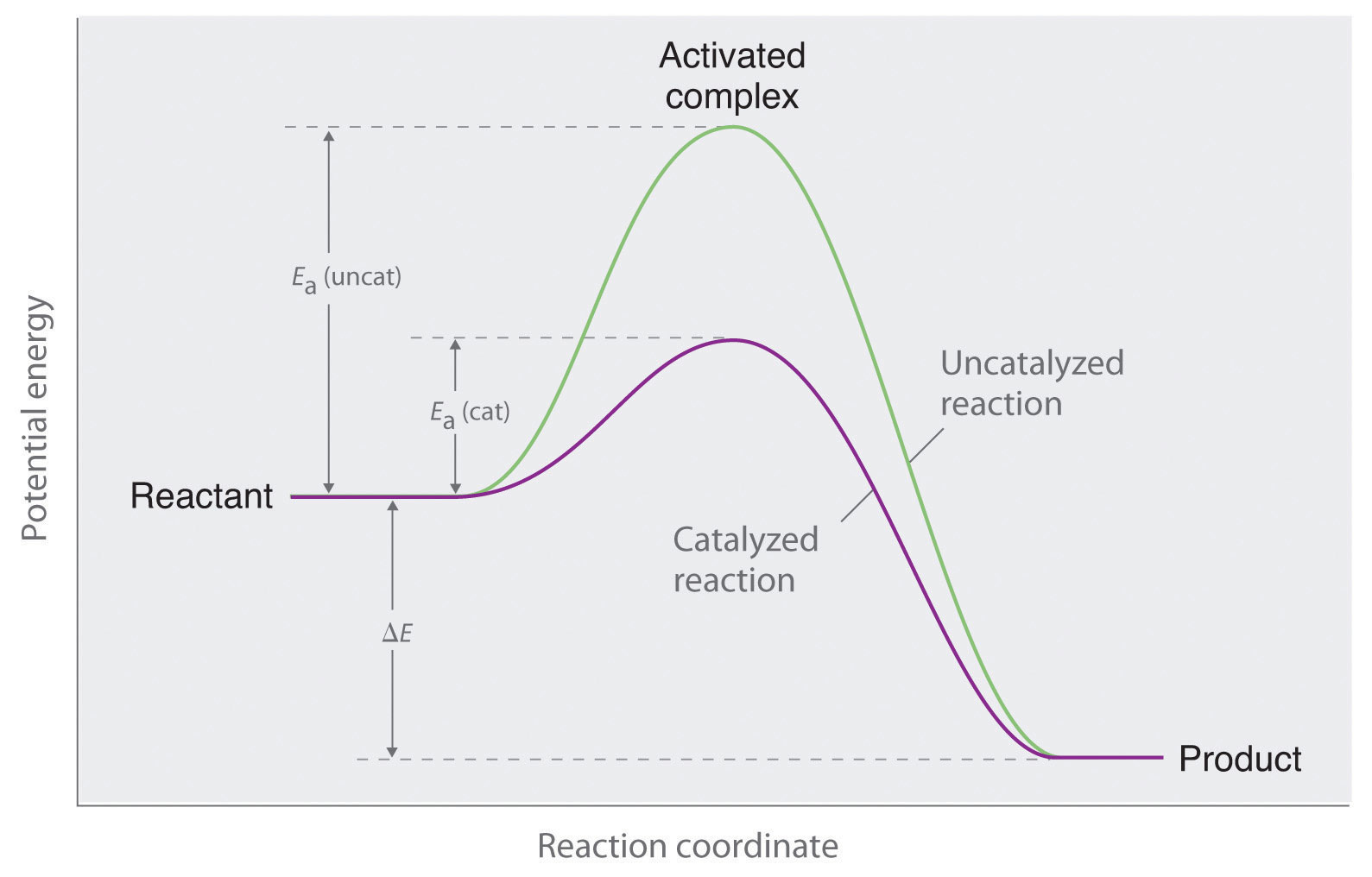
Catalyst Position . Explain the function of a catalyst in terms of reaction mechanisms and. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. It covers changes to the. By the end of this section, you will be able to: This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst reduces the time taken to. The position of equilibrium is not changed if you add (or change) a catalyst. Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount.
from socratic.org
This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. The position of equilibrium is not changed if you add (or change) a catalyst. A catalyst reduces the time taken to. It covers changes to the. A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. By the end of this section, you will be able to: Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Explain the function of a catalyst in terms of reaction mechanisms and.
What will occur if a catalyst is added to a reaction mixture? Socratic
Catalyst Position A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. The position of equilibrium is not changed if you add (or change) a catalyst. Explain the function of a catalyst in terms of reaction mechanisms and. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. It covers changes to the. By the end of this section, you will be able to: Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. A catalyst reduces the time taken to.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Position A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. A catalyst reduces the time taken to. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst speeds up both the forward and the. Catalyst Position.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Position Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A catalyst reduces the time taken to. Explain the function of a catalyst in terms of reaction mechanisms and. A catalyst. Catalyst Position.
From www.researchgate.net
8 Position dependent solid phase catalyst activity profiles satisfying Catalyst Position The position of equilibrium is not changed if you add (or change) a catalyst. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Explain the function of a catalyst in terms of reaction mechanisms and. By the end of this section, you will be able to: Explanation a. Catalyst Position.
From themotorguy.com
P0420 Catalyst System Efficiency Below Threshold (Bank 1) The Motor Guy Catalyst Position By the end of this section, you will be able to: Explain the function of a catalyst in terms of reaction mechanisms and. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A catalyst is a substance that increases the rate of a reaction, without being used up or. Catalyst Position.
From chem.libretexts.org
12.8 Catalysis Chemistry LibreTexts Catalyst Position The position of equilibrium is not changed if you add (or change) a catalyst. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. It covers changes to the. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. This page. Catalyst Position.
From www.researchgate.net
4 Effect of catalyst position and catalytic layer thickness on module Catalyst Position A catalyst reduces the time taken to. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The position of equilibrium is not changed if you add (or change) a catalyst. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of. Catalyst Position.
From www.mdpi.com
Catalysts Free FullText A Thermally Conductive Pt/AAO Catalyst for Catalyst Position A catalyst reduces the time taken to. The position of equilibrium is not changed if you add (or change) a catalyst. A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. A catalyst speeds up both the forward and the reverse reactions, so there is. Catalyst Position.
From www.cell.com
Photochlorination of linear alkanes with 2position selectivity using a Catalyst Position Explain the function of a catalyst in terms of reaction mechanisms and. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. A catalyst reduces the time taken to. Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. It covers changes to. Catalyst Position.
From www.numerade.com
SOLVEDAssume that the following exothermic reaction is at equilibrium Catalyst Position A catalyst reduces the time taken to. The position of equilibrium is not changed if you add (or change) a catalyst. A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. By the end of this section, you will be able to: Le chatelier's principle. Catalyst Position.
From mungfali.com
IDEXX Catalyst One Catalyst Position Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. The position of equilibrium is not changed if you add (or change) a catalyst. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Catalysis is the ability of some species to rapidly speed. Catalyst Position.
From www.researchgate.net
IR camera temperature map of Pt/AAO catalyst obtained at steadystate Catalyst Position A catalyst reduces the time taken to. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. A catalyst speeds up both the forward and the reverse reactions, so. Catalyst Position.
From www.behance.net
Catalytic Converter Vector Illustration Behance Catalyst Position Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. The position of equilibrium is not changed if you add (or change) a catalyst. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst speeds up both the forward and the reverse reactions,. Catalyst Position.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.3.3 The Position of Equilibrium Catalyst Position Explain the function of a catalyst in terms of reaction mechanisms and. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. It covers changes to the. A catalyst reduces the time taken to. A catalyst is a substance that increases the rate of a reaction, without being used. Catalyst Position.
From www.researchgate.net
6 Effect of catalyst position and catalytic layer thickness on module Catalyst Position The position of equilibrium is not changed if you add (or change) a catalyst. It covers changes to the. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst. Catalyst Position.
From www.researchgate.net
shows that the catalyst position behind the ceramic piece has a little Catalyst Position The position of equilibrium is not changed if you add (or change) a catalyst. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. By the end of this section, you will be able to: This page looks at le chatelier's principle and explains how to apply it to reactions in. Catalyst Position.
From www.marketbeat.com
Friedenthal Financial Takes Position in Catalyst Pharmaceuticals, Inc Catalyst Position A catalyst reduces the time taken to. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A catalyst speeds up both the forward and the reverse reactions, so. Catalyst Position.
From www.researchgate.net
Schematic illustration showing the strategies to improve the catalytic Catalyst Position Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates.. Catalyst Position.
From www.researchgate.net
CBS reductions employing modified catalysts with DEDs in the catalyst's Catalyst Position By the end of this section, you will be able to: A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. It covers changes to the. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction. Catalyst Position.
From www.mdpi.com
Catalysts Free FullText A Thermally Conductive Pt/AAO Catalyst for Catalyst Position Explain the function of a catalyst in terms of reaction mechanisms and. Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. The position of equilibrium is not changed if you add (or change) a catalyst. A catalyst is a substance that increases the rate of a reaction, without being used up or changed. Catalyst Position.
From blog.olx.com.pk
Everything You Need to Know About Catalytic Converters Catalyst Position By the end of this section, you will be able to: Explain the function of a catalyst in terms of reaction mechanisms and. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change. Catalyst Position.
From www.researchgate.net
Schematic of catalyst coated‐substrate (CCS) and catalyst coated Catalyst Position Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. Explain the function of a catalyst in terms of reaction mechanisms and. This page looks at le chatelier's principle and explains. Catalyst Position.
From www.researchgate.net
Schematic of the catalyst position P1 (top) and P2 (bottom). In both Catalyst Position The position of equilibrium is not changed if you add (or change) a catalyst. By the end of this section, you will be able to: Explain the function of a catalyst in terms of reaction mechanisms and. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Explanation a catalyst. Catalyst Position.
From slideplayer.com
Factors That Affect Equilibrium ppt download Catalyst Position Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The. Catalyst Position.
From professional.patrickneyman.com
Activation Energy Equation Catalyst Position By the end of this section, you will be able to: A catalyst reduces the time taken to. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. This page looks. Catalyst Position.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.21C Understand Why a Catalyst Does Not Affect Catalyst Position This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. It covers changes to the. A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. Explanation a catalyst speeds up both the forward and. Catalyst Position.
From socratic.org
What will occur if a catalyst is added to a reaction mixture? Socratic Catalyst Position A catalyst reduces the time taken to. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. This page looks at le chatelier's principle and explains how to apply it. Catalyst Position.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Position Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven. Catalyst Position.
From www.researchgate.net
(a) HAADF image of a nanowire apex. (b) Schematics of the catalyst Catalyst Position Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. A catalyst reduces the time taken to. By the end of this section, you will be able to: A. Catalyst Position.
From www.mdpi.com
Catalysts Free FullText A Thermally Conductive Pt/AAO Catalyst for Catalyst Position It covers changes to the. A catalyst reduces the time taken to. Explain the function of a catalyst in terms of reaction mechanisms and. Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic. Catalyst Position.
From www.carparts.com
Where is the Catalytic Converter Located? In The Garage with Catalyst Position A catalyst reduces the time taken to. By the end of this section, you will be able to: Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. This page looks at le chatelier's principle. Catalyst Position.
From www.mdpi.com
Catalysts Free FullText FCC Catalyst Accessibility—A Review Catalyst Position A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. By the end of this section, you will be able to: Catalysis is the ability. Catalyst Position.
From www.mdpi.com
Catalysts Free FullText A Thermally Conductive Pt/AAO Catalyst for Catalyst Position Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. A catalyst is a substance that increases the rate of a reaction, without being used up or changed chemically by the end of the reaction. By the end of this section, you will be able to: This page looks at le chatelier's principle and. Catalyst Position.
From www.researchgate.net
Possible position ofSCR catalyst installation Download Scientific Diagram Catalyst Position By the end of this section, you will be able to: A catalyst reduces the time taken to. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. This page looks. Catalyst Position.
From www.researchgate.net
Linescan in RC mode for evaluating the position of center of the Catalyst Position A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Explain the function of a catalyst in terms of reaction mechanisms and. Explanation a catalyst speeds up both the forward and back reactions by exactly the same amount. It covers changes to the. Catalysis is the ability of some species. Catalyst Position.
From www.researchgate.net
Schematic diagram of different plasmacatalyst configurations according Catalyst Position By the end of this section, you will be able to: This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Catalysis is the ability of some species to rapidly speed up the rate at which a chemical reaction proceeds. A catalyst reduces the time taken to. A catalyst. Catalyst Position.