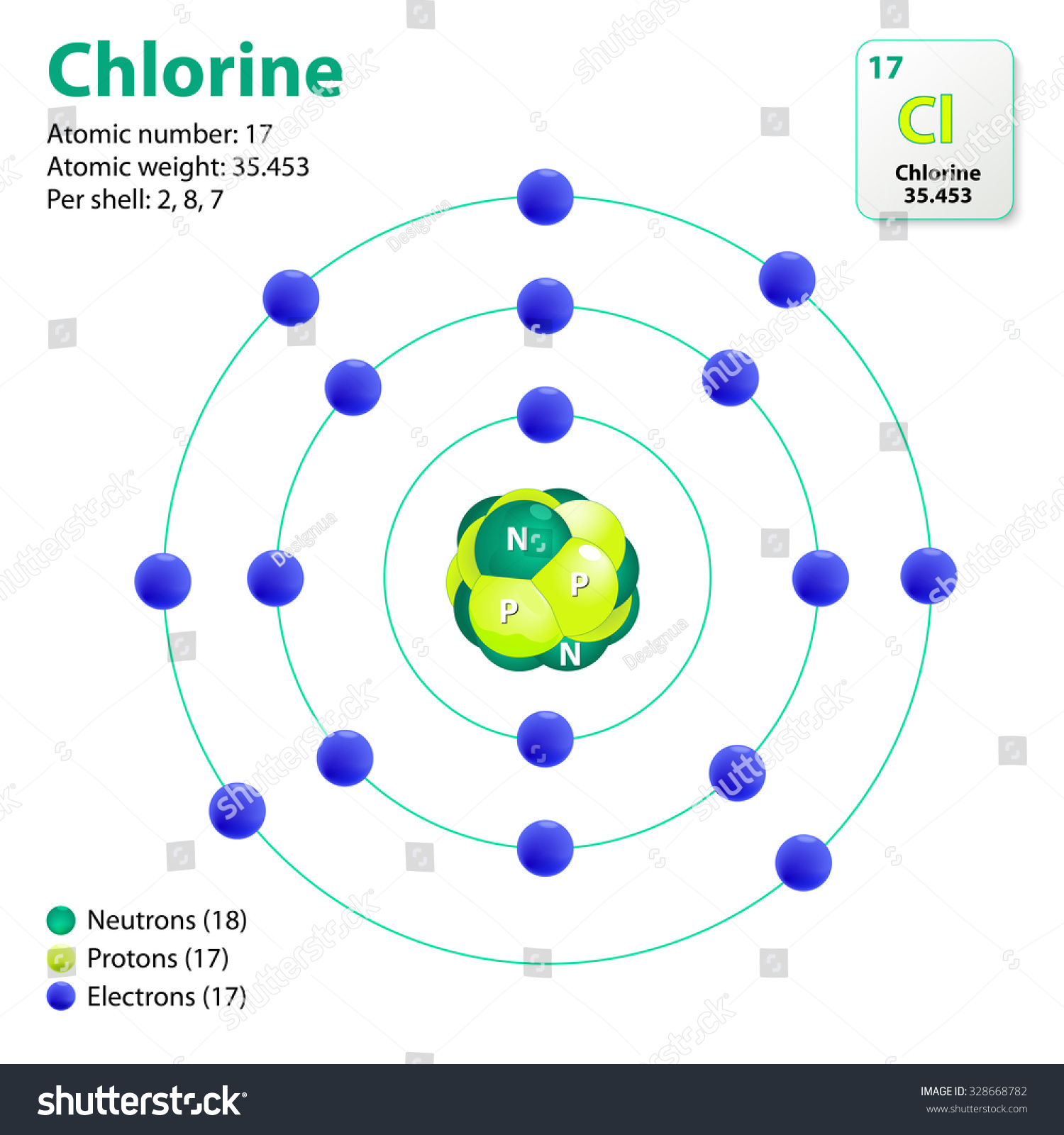
Chlorine Atom Needs One Electron . Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. Thus we need two cl atoms. A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. This makes chlorine a #cl^(−1)#. A neutral chlorine atom has seven electrons in its outermost shell. Chlorine has 7 valence electrons. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Only one more electron is needed to achieve an octet in. Since it has 1 more electron than protons, chlorine has a charge of. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? It needs one electron to make it stable at 8 electrons in its valence shells. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5.
from drivenheisenberg.blogspot.com
A neutral chlorine atom has seven electrons in its outermost shell. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Thus we need two cl atoms. It needs one electron to make it stable at 8 electrons in its valence shells. A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? Since it has 1 more electron than protons, chlorine has a charge of. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. This makes chlorine a #cl^(−1)#.
Electron Dot Diagram For Chlorine Drivenheisenberg
Chlorine Atom Needs One Electron Since it has 1 more electron than protons, chlorine has a charge of. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Only one more electron is needed to achieve an octet in. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Chlorine has 7 valence electrons. Since it has 1 more electron than protons, chlorine has a charge of. A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. It needs one electron to make it stable at 8 electrons in its valence shells. This makes chlorine a #cl^(−1)#. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? Thus we need two cl atoms. A neutral chlorine atom has seven electrons in its outermost shell.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Atom Needs One Electron The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Chlorine has 7 valence electrons. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. It needs. Chlorine Atom Needs One Electron.
From slideplayer.com
Bonding. ppt download Chlorine Atom Needs One Electron Thus we need two cl atoms. Chlorine has 7 valence electrons. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? Since it has 1 more electron than protons, chlorine has a charge of. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving. Chlorine Atom Needs One Electron.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Atom Needs One Electron Chlorine has 7 valence electrons. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. This makes chlorine a #cl^(−1)#. Since it has 1 more electron than protons, chlorine has a charge of. It needs one electron to make it stable at 8 electrons in its valence shells. Only one more. Chlorine Atom Needs One Electron.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Chlorine Atom Needs One Electron From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. It needs one electron to make it stable at 8 electrons in its valence shells. A cl atom needs only one more to complete its octet,. Chlorine Atom Needs One Electron.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Atom Needs One Electron • chlorine gains an electron, leaving it with 17 protons and 18 electrons. A neutral chlorine atom has seven electrons in its outermost shell. This makes chlorine a #cl^(−1)#. It needs one electron to make it stable at 8 electrons in its valence shells. A cl atom needs only one more to complete its octet, while ca atoms have two. Chlorine Atom Needs One Electron.
From www.pinterest.co.uk
chlorine Electron configuration, Atom model project, Atom model Chlorine Atom Needs One Electron This makes chlorine a #cl^(−1)#. Only one more electron is needed to achieve an octet in. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? Thus we need two cl atoms. Since it has 1 more electron than protons, chlorine has a charge of.. Chlorine Atom Needs One Electron.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science Chlorine Atom Needs One Electron • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Since it has 1 more electron than protons, chlorine has a charge of. Chlorine has the electron configuration. Chlorine Atom Needs One Electron.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Atom Needs One Electron Only one more electron is needed to achieve an octet in. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. Thus we need two cl atoms. A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. Chlorine has 7 valence electrons.. Chlorine Atom Needs One Electron.
From slideplayer.com
Chemical Bonds & Reactions ppt download Chlorine Atom Needs One Electron A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. Only one more electron is needed to achieve an octet in. Thus we need two cl atoms. Since it has 1 more electron than protons, chlorine has a charge of. • chlorine gains an electron, leaving it with 17 protons and. Chlorine Atom Needs One Electron.
From gioqzurub.blob.core.windows.net
Chlorine Atom Electron Dot Structure at Omar Turk blog Chlorine Atom Needs One Electron From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? Since it has 1 more electron than protons, chlorine has a charge of. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Thus we need two cl atoms. This makes chlorine. Chlorine Atom Needs One Electron.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Atom Needs One Electron A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. A neutral chlorine atom has seven electrons in its outermost shell. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many. Chlorine Atom Needs One Electron.
From www.mooramo.com
The Formation of Ionic Compounds From Atoms Mooramo Chlorine Atom Needs One Electron Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. It needs one electron to make it stable at 8 electrons in its valence shells. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. A neutral chlorine atom has seven electrons in its outermost shell. The electron. Chlorine Atom Needs One Electron.
From mammothmemory.net
A certain amount of electrons are allowed in certain shells Chlorine Atom Needs One Electron This makes chlorine a #cl^(−1)#. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? Only one more electron is needed to achieve an octet in. Chlorine has 7 valence electrons. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence. Chlorine Atom Needs One Electron.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Atom Needs One Electron It needs one electron to make it stable at 8 electrons in its valence shells. A neutral chlorine atom has seven electrons in its outermost shell. Thus we need two cl atoms. A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. Since it has 1 more electron than protons, chlorine. Chlorine Atom Needs One Electron.
From www.dreamstime.com
Chlorine Atom, with Mass and Energy Levels. Stock Vector Illustration Chlorine Atom Needs One Electron Thus we need two cl atoms. This makes chlorine a #cl^(−1)#. Since it has 1 more electron than protons, chlorine has a charge of. It needs one electron to make it stable at 8 electrons in its valence shells. Only one more electron is needed to achieve an octet in. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)),. Chlorine Atom Needs One Electron.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Chlorine Atom Needs One Electron Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine has 7 valence electrons. Since it has 1 more electron than protons, chlorine has a charge of. • chlorine gains an electron, leaving it with. Chlorine Atom Needs One Electron.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains Chlorine Atom Needs One Electron This makes chlorine a #cl^(−1)#. Since it has 1 more electron than protons, chlorine has a charge of. It needs one electron to make it stable at 8 electrons in its valence shells. Thus we need two cl atoms. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. The electron configuration of chlorine is 1s22s22p63s23p5 or. Chlorine Atom Needs One Electron.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Atom Needs One Electron Since it has 1 more electron than protons, chlorine has a charge of. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. Chlorine has 7 valence electrons. This makes chlorine a #cl^(−1)#. A cl atom needs only. Chlorine Atom Needs One Electron.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Atom Needs One Electron From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? Thus we need two cl atoms. A neutral chlorine atom has seven electrons in its outermost shell. Since it has 1 more electron than protons, chlorine has a charge of. The electron configuration of chlorine. Chlorine Atom Needs One Electron.
From www.numerade.com
SOLVEDChlorine tends to form only one covalent bond because it needs Chlorine Atom Needs One Electron Only one more electron is needed to achieve an octet in. This makes chlorine a #cl^(−1)#. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. It needs one electron to make it stable at 8 electrons in its valence shells. A cl atom needs only one more to complete its octet, while ca atoms have two. Chlorine Atom Needs One Electron.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Atom Needs One Electron Thus we need two cl atoms. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. It needs one electron to make it stable at 8 electrons in its valence shells. Only one more electron is needed to. Chlorine Atom Needs One Electron.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Atom Needs One Electron This makes chlorine a #cl^(−1)#. A neutral chlorine atom has seven electrons in its outermost shell. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. It needs one electron to make it stable at 8 electrons in its valence shells. Thus we need two cl atoms. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. From. Chlorine Atom Needs One Electron.
From www.shutterstock.com
Bohr Model Chlorine Atom Electron Structure Stock Vector (Royalty Free Chlorine Atom Needs One Electron Only one more electron is needed to achieve an octet in. Thus we need two cl atoms. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. Since it has 1 more electron than protons, chlorine has a charge of. This makes chlorine a #cl^(−1)#. From the electron configuration of neutral. Chlorine Atom Needs One Electron.
From www.numerade.com
SOLVED Use the element symbols to draw in the ekctronic configuration Chlorine Atom Needs One Electron Only one more electron is needed to achieve an octet in. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? • chlorine gains an electron, leaving it with 17 protons and 18 electrons. A cl atom needs only one more to complete its octet,. Chlorine Atom Needs One Electron.
From quizlet.com
Diagram the atomic structure of atomic number i Quizlet Chlorine Atom Needs One Electron From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. It needs one electron to make it stable at 8 electrons in its valence shells. •. Chlorine Atom Needs One Electron.
From gioqzurub.blob.core.windows.net
Chlorine Atom Electron Dot Structure at Omar Turk blog Chlorine Atom Needs One Electron • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Only one more electron is needed to achieve an octet in. A neutral chlorine atom has seven electrons in its outermost shell. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have?. Chlorine Atom Needs One Electron.
From slideplayer.com
Bonding. ppt download Chlorine Atom Needs One Electron The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. This makes chlorine a #cl^(−1)#. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence. Chlorine Atom Needs One Electron.
From www.shutterstock.com
Bohr Model Chlorine Atom Electron Structure vetor stock (livre de Chlorine Atom Needs One Electron A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. This makes chlorine a #cl^(−1)#. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? It needs. Chlorine Atom Needs One Electron.
From www.numerade.com
SOLVED What change is occurring in this figure? Nat Sodium atom Chlorine Atom Needs One Electron The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. A cl atom needs only one more to complete its octet, while ca atoms have two electrons to lose. It needs one electron to make it stable at 8 electrons in its valence shells. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Chlorine has 7 valence. Chlorine Atom Needs One Electron.
From gardenandplate.com
Molecules Chlorine Atom Needs One Electron • chlorine gains an electron, leaving it with 17 protons and 18 electrons. From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Only one more electron is needed to achieve an octet in. Since it. Chlorine Atom Needs One Electron.
From gioqzurub.blob.core.windows.net
Chlorine Atom Electron Dot Structure at Omar Turk blog Chlorine Atom Needs One Electron From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? This makes chlorine a #cl^(−1)#. It needs one electron to make it stable at 8 electrons in its valence shells. A neutral chlorine atom has seven electrons in its outermost shell. Chlorine has the electron. Chlorine Atom Needs One Electron.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Chlorine Atom Needs One Electron A neutral chlorine atom has seven electrons in its outermost shell. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Since it has 1 more electron than protons, chlorine has a charge of. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Chlorine has 7 valence electrons. Only one more electron is needed to achieve an. Chlorine Atom Needs One Electron.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Atom Needs One Electron The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Only one more electron is needed to achieve an octet in. • chlorine gains an electron, leaving it with 17 protons and 18 electrons. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. A neutral chlorine atom has seven electrons in. Chlorine Atom Needs One Electron.
From opentextbc.ca
2.2 Bonding and Lattices Physical Geology Chlorine Atom Needs One Electron Since it has 1 more electron than protons, chlorine has a charge of. A neutral chlorine atom has seven electrons in its outermost shell. Chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. It needs one electron to make it stable at 8 electrons in its valence shells. From the. Chlorine Atom Needs One Electron.
From drivenheisenberg.blogspot.com
Electron Dot Diagram For Chlorine Drivenheisenberg Chlorine Atom Needs One Electron From the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a neutral chlorine atom have? • chlorine gains an electron, leaving it with 17 protons and 18 electrons. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Since it has 1 more electron than protons, chlorine has a charge of.. Chlorine Atom Needs One Electron.