What Is Mdr For Medical Device . The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. This document supersedes “medical device reporting for manufacturers” dated march 1997. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. This guidance represents the current thinking of.
from www.mantrasystems.co.uk
In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. This guidance represents the current thinking of. This document supersedes “medical device reporting for manufacturers” dated march 1997.
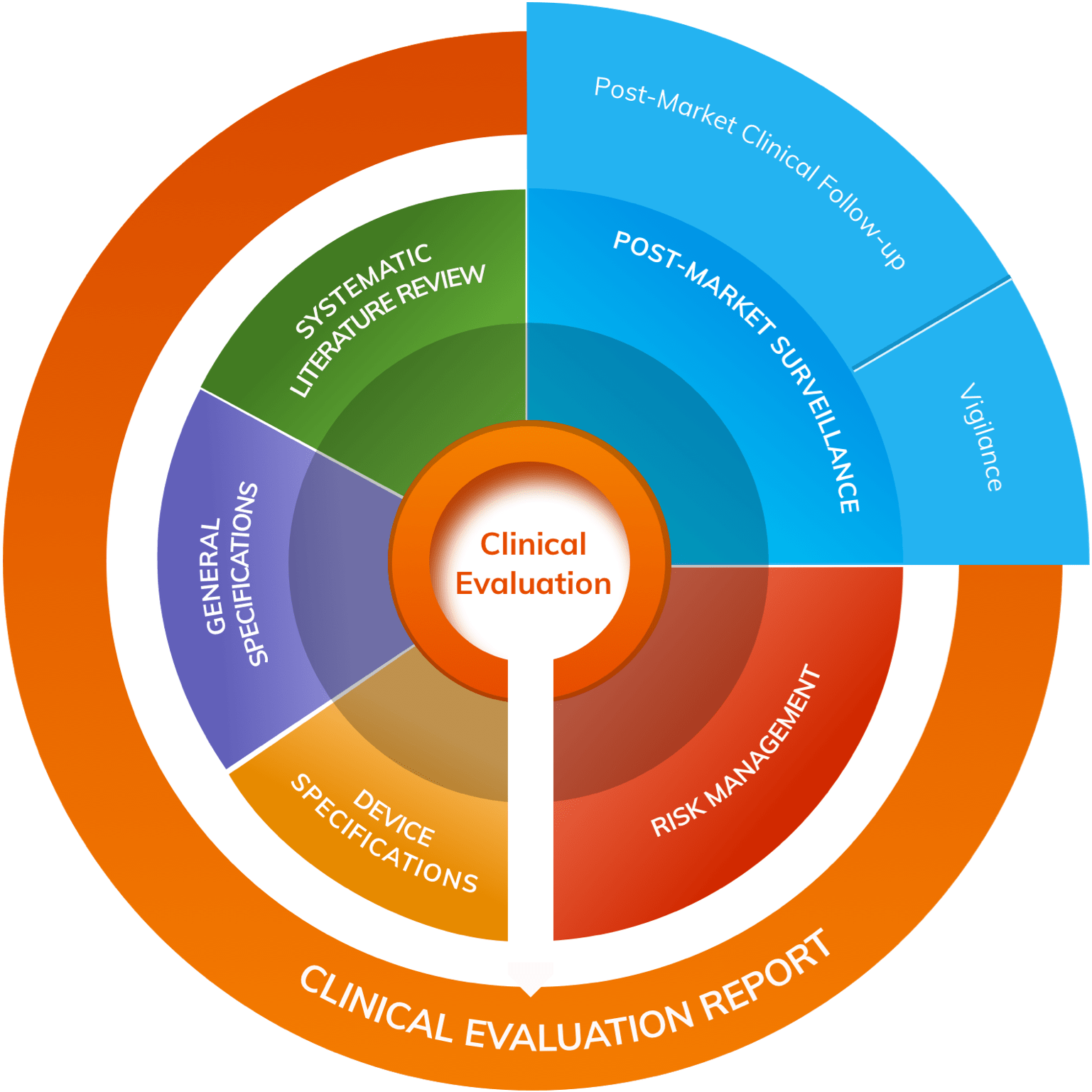
EU MDR Clinical Evaluation for Medical Device Approval
What Is Mdr For Medical Device The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. This guidance represents the current thinking of. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. This document supersedes “medical device reporting for manufacturers” dated march 1997. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF What Is Mdr For Medical Device This guidance represents the current thinking of. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In the european union (eu), the regulation of medical. What Is Mdr For Medical Device.
From www.medtextpert.com
8 Facts Every Medical Software Developer Should Know About the MDR What Is Mdr For Medical Device This guidance represents the current thinking of. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. This document supersedes “medical device reporting for. What Is Mdr For Medical Device.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide What Is Mdr For Medical Device The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. This document supersedes “medical device reporting for manufacturers” dated march 1997. In the european union (eu), the regulation of medical devices has undergone a significant. What Is Mdr For Medical Device.
From www.mantrasystems.co.uk
Software as a Medical Device (SaMD) for the EU MDR What Is Mdr For Medical Device In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. This guidance represents the current thinking of. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part. What Is Mdr For Medical Device.
From tsquality.ch
MDR Classification Document TSQuality.ch What Is Mdr For Medical Device The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. This guidance represents the current thinking of. The medical device reporting (mdr) regulation (21 cfr. What Is Mdr For Medical Device.
From medicaldevicehq.com
Medical Device Regulation codes Medical Device HQ 1 What Is Mdr For Medical Device This guidance represents the current thinking of. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The european medical devices regulation, (eu) 2017/745 (mdr), replaces. What Is Mdr For Medical Device.
From decomplix.com
Medical device software (MDSW) under EU MDR and IVDR What Is Mdr For Medical Device The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. This guidance represents the current thinking of. This document supersedes “medical device reporting for manufacturers” dated march 1997. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as. What Is Mdr For Medical Device.
From galtmedical.com
What the EU MDR Extension Means for Medical Device Buyers Galt Medical What Is Mdr For Medical Device This document supersedes “medical device reporting for manufacturers” dated march 1997. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. This guidance represents the current thinking of. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd). What Is Mdr For Medical Device.
From medicaliomt.com
What Is MDR In Medical Technology Medical IoMT What Is Mdr For Medical Device The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes. What Is Mdr For Medical Device.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is Mdr For Medical Device In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. This document supersedes “medical device reporting for manufacturers” dated march 1997. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device. What Is Mdr For Medical Device.
From citemedical.com
Medical Device Reporting (MDR) How to Report Problems to the FDA What Is Mdr For Medical Device The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. This guidance represents the current thinking of. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers,. What Is Mdr For Medical Device.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF What Is Mdr For Medical Device In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. This document supersedes “medical device reporting for manufacturers” dated march 1997. The medical device regulation (mdr), which. What Is Mdr For Medical Device.
From medicaldevicehq.com
What is a medical device according to the MDR Medical Device HQ 1 What Is Mdr For Medical Device The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. This guidance represents the current thinking of. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework. What Is Mdr For Medical Device.
From www.youtube.com
What is MDR? Medical Device Regulation Introductory Training YouTube What Is Mdr For Medical Device This document supersedes “medical device reporting for manufacturers” dated march 1997. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. This guidance represents the current thinking of. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the european union (eu),. What Is Mdr For Medical Device.
From intellisoft.io
EU Regulation Transitioning from the MDD to MDR What Is Mdr For Medical Device This document supersedes “medical device reporting for manufacturers” dated march 1997. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. The european medical devices regulation, (eu). What Is Mdr For Medical Device.
From www.greenlight.guru
Medical Device Reporting (MDR) How to Take Advantage of Your What Is Mdr For Medical Device In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. This document supersedes “medical device reporting for. What Is Mdr For Medical Device.
From www.universalmedica.com
Key Aspects of New EU Medical Devices Regulation (EU 2017/745 What Is Mdr For Medical Device This guidance represents the current thinking of. This document supersedes “medical device reporting for manufacturers” dated march 1997. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the mdr, a medical. What Is Mdr For Medical Device.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX What Is Mdr For Medical Device The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. This document supersedes “medical device reporting for manufacturers” dated march 1997. This guidance represents the current thinking of. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical. What Is Mdr For Medical Device.
From cliniexperts.com
Medical Device Grouping as per MDR 2017 CliniExperts CliniExperts What Is Mdr For Medical Device In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active. What Is Mdr For Medical Device.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I What Is Mdr For Medical Device In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. This document supersedes “medical device reporting for manufacturers” dated march 1997. The medical. What Is Mdr For Medical Device.
From www.mantrasystems.co.uk
EU MDR Clinical Evaluation for Medical Device Approval What Is Mdr For Medical Device This document supersedes “medical device reporting for manufacturers” dated march 1997. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. The medical device. What Is Mdr For Medical Device.
From pharmait.dk
Concept, Of, Mdr, Medical, Device, Regulation. Pharma IT What Is Mdr For Medical Device The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. This document supersedes “medical device reporting for manufacturers” dated march 1997. In the mdr, a medical device is stated in article 2, “definitions”,. What Is Mdr For Medical Device.
From operonstrategist.com
Simplify CE Marking with MDR Technical Documentation Support What Is Mdr For Medical Device This guidance represents the current thinking of. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. In the european union (eu), the. What Is Mdr For Medical Device.
From custom-medical.com
MDR What changes for medical device manufacturers Custom Medical What Is Mdr For Medical Device The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. This document supersedes “medical device reporting for manufacturers” dated march 1997. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the mdr, a medical device is stated in article 2, “definitions”,. What Is Mdr For Medical Device.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU What Is Mdr For Medical Device This guidance represents the current thinking of. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european medical devices regulation, (eu) 2017/745 (mdr),. What Is Mdr For Medical Device.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is Mdr For Medical Device The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. This guidance represents the current thinking of. In the mdr, a medical device is stated in article 2, “definitions”, as the very first. What Is Mdr For Medical Device.
From luzernbaar.ch
Are you ready for the MDR? This is how new EU regulations may impact What Is Mdr For Medical Device This document supersedes “medical device reporting for manufacturers” dated march 1997. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes. What Is Mdr For Medical Device.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft What Is Mdr For Medical Device This document supersedes “medical device reporting for manufacturers” dated march 1997. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. In the european union (eu), the regulation of medical devices has. What Is Mdr For Medical Device.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 What Is Mdr For Medical Device The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. This guidance represents the current. What Is Mdr For Medical Device.
From www.haredataelectronics.co.uk
What is MDR? The New Medical Device Regulation What Is Mdr For Medical Device The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. This guidance represents the current thinking of. This document supersedes “medical device reporting for manufacturers” dated march 1997. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd). What Is Mdr For Medical Device.
From www.iascertification.com
EUMDR Certification Medical Device Regulation IAS What Is Mdr For Medical Device This guidance represents the current thinking of. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. This document supersedes “medical device reporting. What Is Mdr For Medical Device.
From www.tuvsud.com
New Medical Device Regulation TÜV SÜD What Is Mdr For Medical Device In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. This guidance represents the current thinking of.. What Is Mdr For Medical Device.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF What Is Mdr For Medical Device This document supersedes “medical device reporting for manufacturers” dated march 1997. In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes. What Is Mdr For Medical Device.
From qbdgroup.com
How to Plan MDR Compliance for Your Medical Device? What Is Mdr For Medical Device In the european union (eu), the regulation of medical devices has undergone a significant transformation with the transition from the medical device directive (mdd) to the. This document supersedes “medical device reporting for manufacturers” dated march 1997. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device. What Is Mdr For Medical Device.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements What Is Mdr For Medical Device In the mdr, a medical device is stated in article 2, “definitions”, as the very first definition and it includes specific medical purposes such as diagnosis, monitoring,. This guidance represents the current thinking of. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device regulation (mdr), which. What Is Mdr For Medical Device.