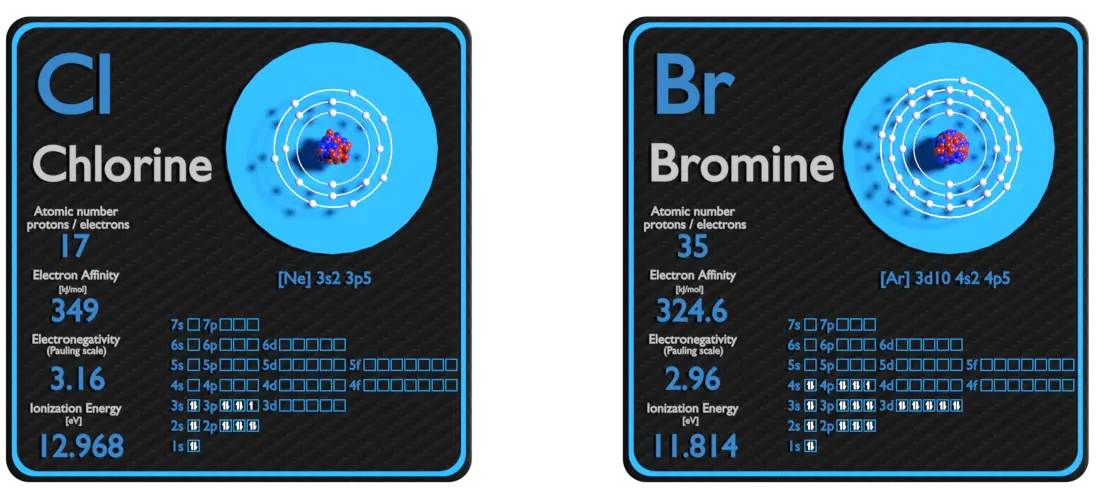
Electronegativity Bromine Chlorine . The concept of electronegativity is originated from the experimental observation, for. 3.16 is the electronegativity value of chlorine (cl). Electronegativity is used to predict whether a bond between atoms will be. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Any shifts in electron density are too small to be noticed and the electron. Polar covalent bonds a bond. 119 rows values for electronegativity run from 0 to 4. what is electronegativity?
from material-properties.org
Electronegativity is used to predict whether a bond between atoms will be. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The concept of electronegativity is originated from the experimental observation, for. 119 rows values for electronegativity run from 0 to 4. Polar covalent bonds a bond. 3.16 is the electronegativity value of chlorine (cl). Any shifts in electron density are too small to be noticed and the electron. what is electronegativity?
Chlorine and Bromine Comparison Properties Material Properties
Electronegativity Bromine Chlorine Polar covalent bonds a bond. 119 rows values for electronegativity run from 0 to 4. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. what is electronegativity? Any shifts in electron density are too small to be noticed and the electron. The concept of electronegativity is originated from the experimental observation, for. Polar covalent bonds a bond. Electronegativity is used to predict whether a bond between atoms will be. 3.16 is the electronegativity value of chlorine (cl).
From www.chegg.com
Solved Question Bromine is less electronegative than Electronegativity Bromine Chlorine Electronegativity is used to predict whether a bond between atoms will be. The concept of electronegativity is originated from the experimental observation, for. what is electronegativity? Polar covalent bonds a bond. 119 rows values for electronegativity run from 0 to 4. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd. Electronegativity Bromine Chlorine.
From learnah.org
AQA Jun 2016 Paper 2 Q6 (with explained solutions) Electronegativity Bromine Chlorine Polar covalent bonds a bond. 119 rows values for electronegativity run from 0 to 4. Any shifts in electron density are too small to be noticed and the electron. Electronegativity is used to predict whether a bond between atoms will be. what is electronegativity? It belongs to the 7th group and 2nd period on the periodic table, known. Electronegativity Bromine Chlorine.
From blog.naver.com
Electronegativity table, 전기음성도 표 네이버 블로그 Electronegativity Bromine Chlorine what is electronegativity? Any shifts in electron density are too small to be noticed and the electron. The concept of electronegativity is originated from the experimental observation, for. Electronegativity is used to predict whether a bond between atoms will be. 3.16 is the electronegativity value of chlorine (cl). 119 rows values for electronegativity run from 0 to. Electronegativity Bromine Chlorine.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Electronegativity Bromine Chlorine It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows values for electronegativity run from 0 to 4. what is electronegativity? Any shifts in electron density are too small to be noticed and the electron. The concept of electronegativity is originated from the experimental observation, for. Electronegativity is used. Electronegativity Bromine Chlorine.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Electronegativity Bromine Chlorine 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be. what is electronegativity? The concept of electronegativity is originated from the experimental observation, for. 3.16 is the electronegativity value of chlorine (cl). Polar covalent bonds a bond. Any shifts in electron density are too small. Electronegativity Bromine Chlorine.
From es.dreamstime.com
Tabla Periódica Del Electronegativity Imagen de archivo Imagen 38153931 Electronegativity Bromine Chlorine The concept of electronegativity is originated from the experimental observation, for. Any shifts in electron density are too small to be noticed and the electron. 3.16 is the electronegativity value of chlorine (cl). Polar covalent bonds a bond. 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms. Electronegativity Bromine Chlorine.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Electronegativity Bromine Chlorine Any shifts in electron density are too small to be noticed and the electron. 3.16 is the electronegativity value of chlorine (cl). what is electronegativity? It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The concept of electronegativity is originated from the experimental observation, for. Electronegativity is used to predict. Electronegativity Bromine Chlorine.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Electronegativity Bromine Chlorine The concept of electronegativity is originated from the experimental observation, for. 119 rows values for electronegativity run from 0 to 4. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. what is electronegativity? Polar covalent bonds a bond. 3.16 is the electronegativity value of chlorine (cl). Any shifts in. Electronegativity Bromine Chlorine.
From spmchemistry.blog.onlinetuition.com.my
Examples of Redox Reaction Displacement of Halogen SPM Chemistry Electronegativity Bromine Chlorine Polar covalent bonds a bond. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The concept of electronegativity is originated from the experimental observation, for. 119 rows values for electronegativity run from 0 to 4. 3.16 is the electronegativity value of chlorine (cl). what is electronegativity? Any shifts in. Electronegativity Bromine Chlorine.
From www.chegg.com
Solved Which element listed is the most Electronegativity Bromine Chlorine Polar covalent bonds a bond. what is electronegativity? 119 rows values for electronegativity run from 0 to 4. 3.16 is the electronegativity value of chlorine (cl). Any shifts in electron density are too small to be noticed and the electron. Electronegativity is used to predict whether a bond between atoms will be. It belongs to the 7th. Electronegativity Bromine Chlorine.
From www.questionai.com
the chart shows the electroniegativity values as Electronegativity Bromine Chlorine It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). what is electronegativity? 119 rows values for electronegativity run from 0 to 4. The concept of electronegativity is originated from the experimental observation, for. Any shifts in electron density are too small. Electronegativity Bromine Chlorine.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Electronegativity Bromine Chlorine 119 rows values for electronegativity run from 0 to 4. what is electronegativity? It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Electronegativity is used to predict whether a bond between atoms will be. 3.16 is the electronegativity value of chlorine (cl). Polar covalent bonds a bond. The concept. Electronegativity Bromine Chlorine.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Electronegativity Bromine Chlorine It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows values for electronegativity run from 0 to 4. what is electronegativity? 3.16 is the electronegativity value of chlorine (cl). Polar covalent bonds a bond. The concept of electronegativity is originated from the experimental observation, for. Any shifts in. Electronegativity Bromine Chlorine.
From slideplayer.com
Final Review!. ppt download Electronegativity Bromine Chlorine The concept of electronegativity is originated from the experimental observation, for. what is electronegativity? 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Electronegativity is used to predict whether a bond between atoms will be. Any shifts in electron density are too. Electronegativity Bromine Chlorine.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Electronegativity Bromine Chlorine what is electronegativity? 119 rows values for electronegativity run from 0 to 4. 3.16 is the electronegativity value of chlorine (cl). Any shifts in electron density are too small to be noticed and the electron. Polar covalent bonds a bond. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens.. Electronegativity Bromine Chlorine.
From mavink.com
Periodic Table With Electronegativity Values Electronegativity Bromine Chlorine It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The concept of electronegativity is originated from the experimental observation, for. Polar covalent bonds a bond. Electronegativity is used to predict whether a bond between atoms will be. Any shifts in electron density are too small to be noticed and the electron. . Electronegativity Bromine Chlorine.
From learnah.org
OCR A AS Jun 2016 Paper 1 Q23 (with explained solutions) Electronegativity Bromine Chlorine 119 rows values for electronegativity run from 0 to 4. what is electronegativity? It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). Electronegativity is used to predict whether a bond between atoms will be. Any shifts in electron density are too. Electronegativity Bromine Chlorine.
From www.numerade.com
SOLVEDJudging from their relative positions in the Periodic Table Electronegativity Bromine Chlorine Polar covalent bonds a bond. 119 rows values for electronegativity run from 0 to 4. 3.16 is the electronegativity value of chlorine (cl). Electronegativity is used to predict whether a bond between atoms will be. The concept of electronegativity is originated from the experimental observation, for. what is electronegativity? It belongs to the 7th group and 2nd. Electronegativity Bromine Chlorine.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Electronegativity Bromine Chlorine Electronegativity is used to predict whether a bond between atoms will be. what is electronegativity? 119 rows values for electronegativity run from 0 to 4. Any shifts in electron density are too small to be noticed and the electron. The concept of electronegativity is originated from the experimental observation, for. Polar covalent bonds a bond. It belongs to. Electronegativity Bromine Chlorine.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Electronegativity Bromine Chlorine what is electronegativity? Polar covalent bonds a bond. Electronegativity is used to predict whether a bond between atoms will be. 119 rows values for electronegativity run from 0 to 4. Any shifts in electron density are too small to be noticed and the electron. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th. Electronegativity Bromine Chlorine.
From hxerxizou.blob.core.windows.net
Electronegativity Of Chlorine Is Higher Than Sulphur at Krista Electronegativity Bromine Chlorine 119 rows values for electronegativity run from 0 to 4. Any shifts in electron density are too small to be noticed and the electron. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. what is electronegativity? Electronegativity is used to predict. Electronegativity Bromine Chlorine.
From hxecbcbbm.blob.core.windows.net
Is Bromine Electronegative at Isaac Harrell blog Electronegativity Bromine Chlorine what is electronegativity? Polar covalent bonds a bond. The concept of electronegativity is originated from the experimental observation, for. Any shifts in electron density are too small to be noticed and the electron. Electronegativity is used to predict whether a bond between atoms will be. It belongs to the 7th group and 2nd period on the periodic table, known. Electronegativity Bromine Chlorine.
From www.numerade.com
SOLVED DETAILS WAS OSATOMSCHEMI 4.2.WA.013 MY NOTES ASK YOUR TEACHER Electronegativity Bromine Chlorine 119 rows values for electronegativity run from 0 to 4. Polar covalent bonds a bond. The concept of electronegativity is originated from the experimental observation, for. Any shifts in electron density are too small to be noticed and the electron. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on. Electronegativity Bromine Chlorine.
From lessoncampuspolypide.z5.web.core.windows.net
Electronegativity And Polarity Chart Electronegativity Bromine Chlorine what is electronegativity? The concept of electronegativity is originated from the experimental observation, for. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond. Electronegativity Bromine Chlorine.
From www.numerade.com
SOLVED Question 17 (1 point) The interhalogen compound ICl3 can form Electronegativity Bromine Chlorine Any shifts in electron density are too small to be noticed and the electron. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Polar covalent bonds a bond. Electronegativity is used to predict whether a bond between atoms will be. what is electronegativity? 3.16 is the electronegativity value of chlorine. Electronegativity Bromine Chlorine.
From www.trentu.ca
PreChemistry Electronegativity Bromine Chlorine Electronegativity is used to predict whether a bond between atoms will be. 3.16 is the electronegativity value of chlorine (cl). 119 rows values for electronegativity run from 0 to 4. Any shifts in electron density are too small to be noticed and the electron. what is electronegativity? The concept of electronegativity is originated from the experimental observation,. Electronegativity Bromine Chlorine.
From www.numerade.com
SOLVED Arrange the following elements in order of increasing Electronegativity Bromine Chlorine It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. what is electronegativity? Electronegativity is used to predict whether a bond between atoms will be. 3.16 is the electronegativity value of chlorine (cl). Polar covalent bonds a bond. Any shifts in electron density are too small to be noticed and the. Electronegativity Bromine Chlorine.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Electronegativity Bromine Chlorine Polar covalent bonds a bond. The concept of electronegativity is originated from the experimental observation, for. what is electronegativity? Electronegativity is used to predict whether a bond between atoms will be. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119. Electronegativity Bromine Chlorine.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Electronegativity Bromine Chlorine Any shifts in electron density are too small to be noticed and the electron. Polar covalent bonds a bond. 3.16 is the electronegativity value of chlorine (cl). 119 rows values for electronegativity run from 0 to 4. what is electronegativity? The concept of electronegativity is originated from the experimental observation, for. Electronegativity is used to predict whether. Electronegativity Bromine Chlorine.
From www.numerade.com
SOLVED Use the References access important values Visited Arrange the Electronegativity Bromine Chlorine Electronegativity is used to predict whether a bond between atoms will be. The concept of electronegativity is originated from the experimental observation, for. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. what is electronegativity? 119 rows values for electronegativity run from 0 to 4. Polar covalent bonds a bond.. Electronegativity Bromine Chlorine.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Electronegativity Bromine Chlorine Electronegativity is used to predict whether a bond between atoms will be. Any shifts in electron density are too small to be noticed and the electron. what is electronegativity? 3.16 is the electronegativity value of chlorine (cl). 119 rows values for electronegativity run from 0 to 4. It belongs to the 7th group and 2nd period on. Electronegativity Bromine Chlorine.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF Electronegativity Bromine Chlorine The concept of electronegativity is originated from the experimental observation, for. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). what is electronegativity? Polar covalent bonds a bond. 119 rows values for electronegativity run from 0 to 4. Any shifts in. Electronegativity Bromine Chlorine.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Electronegativity Bromine Chlorine Polar covalent bonds a bond. Electronegativity is used to predict whether a bond between atoms will be. 119 rows values for electronegativity run from 0 to 4. Any shifts in electron density are too small to be noticed and the electron. 3.16 is the electronegativity value of chlorine (cl). what is electronegativity? The concept of electronegativity is. Electronegativity Bromine Chlorine.
From byjus.com
why fluorine is more electronegative than oxygen. Electronegativity Bromine Chlorine Electronegativity is used to predict whether a bond between atoms will be. 3.16 is the electronegativity value of chlorine (cl). what is electronegativity? 119 rows values for electronegativity run from 0 to 4. Polar covalent bonds a bond. Any shifts in electron density are too small to be noticed and the electron. The concept of electronegativity is. Electronegativity Bromine Chlorine.