Standard Reduction Potential Sn4+ . identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. 1 m for solutes) usually at 298.15 k;. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Standard reduction potentials by value. Interpret electrode potentials in terms of relative oxidant and reductant strengths. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. The following table provides eo for selected reduction. describe and relate the definitions of electrode and cell potentials. Standard reduction potentials by value. standard reduction potential (e°): The value of the reduction under standard conditions (1 bar or 1 atm for gases;
from www.numerade.com
identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. standard reduction potential (e°): 1 m for solutes) usually at 298.15 k;. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Interpret electrode potentials in terms of relative oxidant and reductant strengths. describe and relate the definitions of electrode and cell potentials. The following table provides eo for selected reduction. Standard reduction potentials by value. The value of the reduction under standard conditions (1 bar or 1 atm for gases;
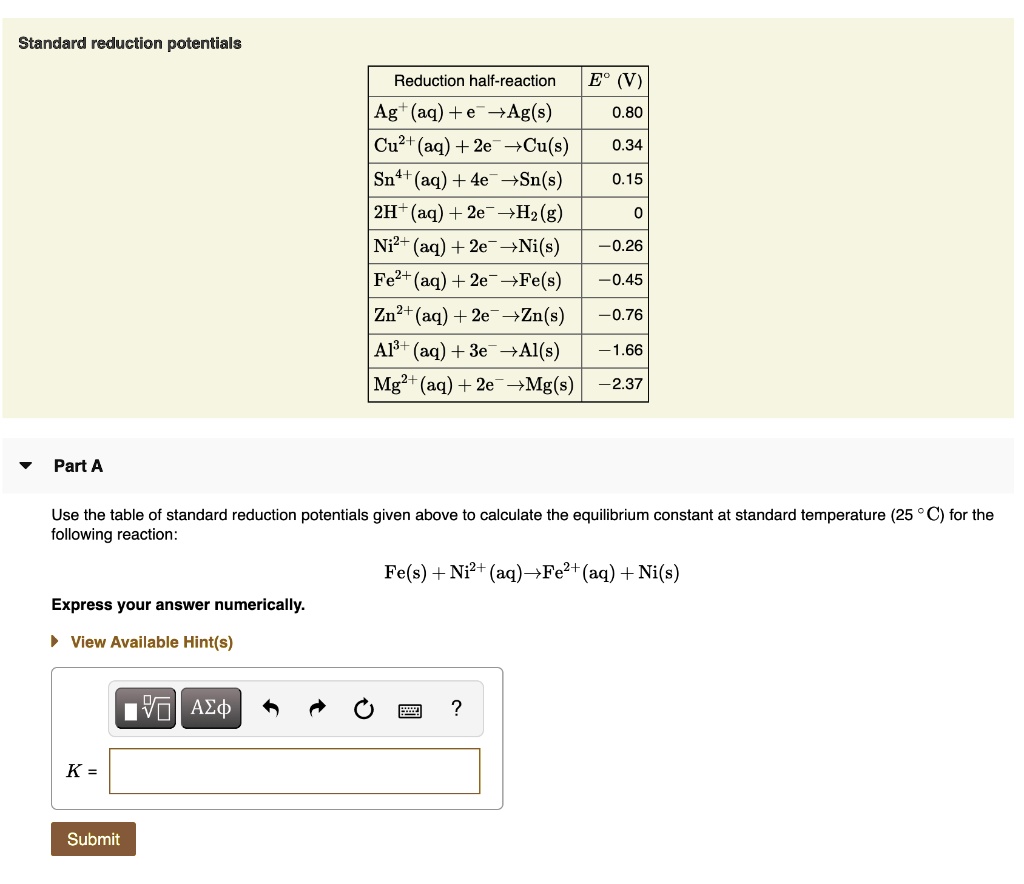
SOLVED Standard reduction potentials Reduction halfreaction Ag(aq
Standard Reduction Potential Sn4+ Interpret electrode potentials in terms of relative oxidant and reductant strengths. standard reduction potential (e°): 1 m for solutes) usually at 298.15 k;. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The value of the reduction under standard conditions (1 bar or 1 atm for gases; identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. The following table provides eo for selected reduction. Standard reduction potentials by value. Standard reduction potentials by value. describe and relate the definitions of electrode and cell potentials.
From www.numerade.com
SOLVED The standard reduction potential of two halfreactions are Standard Reduction Potential Sn4+ The value of the reduction under standard conditions (1 bar or 1 atm for gases; Standard reduction potentials by value. Standard reduction potentials by value. Interpret electrode potentials in terms of relative oxidant and reductant strengths. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. The following table provides eo for selected reduction.. Standard Reduction Potential Sn4+.
From www.bartleby.com
Answered Use standard reduction potentials to… bartleby Standard Reduction Potential Sn4+ a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The following table provides eo for selected reduction. describe and relate the definitions of electrode and cell potentials. standard reduction potential (e°): assigning the potential of. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Selective Reduction The standard reduction potential for the Standard Reduction Potential Sn4+ identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. describe and relate the definitions of electrode and cell potentials. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. a. Standard Reduction Potential Sn4+.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Standard Reduction Potential Sn4+ The value of the reduction under standard conditions (1 bar or 1 atm for gases; assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. standard reduction potential (e°): The following table provides eo for selected reduction. Standard reduction potentials by value. identify how to view standard reduction potentials from. Standard Reduction Potential Sn4+.
From www.youtube.com
Standard Reduction Potential YouTube Standard Reduction Potential Sn4+ assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Standard reduction potentials by value. describe and relate the definitions of electrode and cell potentials. standard reduction potential (e°): The following table provides eo for selected reduction. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value.. Standard Reduction Potential Sn4+.
From www.numerade.com
Which is the stronger reducing agent under standard conditions Sn2 Standard Reduction Potential Sn4+ Interpret electrode potentials in terms of relative oxidant and reductant strengths. The following table provides eo for selected reduction. describe and relate the definitions of electrode and cell potentials. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. assigning the potential of the standard hydrogen electrode (she) as zero volts allows. Standard Reduction Potential Sn4+.
From www.chegg.com
Solved Selective Reduction The standard reduction potential Standard Reduction Potential Sn4+ Standard reduction potentials by value. standard reduction potential (e°): Standard reduction potentials by value. describe and relate the definitions of electrode and cell potentials. The value of the reduction under standard conditions (1 bar or 1 atm for gases; assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. . Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Use the Table of Standard Reduction Potentials to answer the Standard Reduction Potential Sn4+ a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. standard reduction potential (e°): The following table provides eo for selected reduction. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. Interpret electrode potentials in terms of relative oxidant and reductant strengths.. Standard Reduction Potential Sn4+.
From questions-in.kunduz.com
23. The standard reduction potentials Eº Physical Chemistry Standard Reduction Potential Sn4+ Interpret electrode potentials in terms of relative oxidant and reductant strengths. The following table provides eo for selected reduction. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. describe and relate the definitions of electrode and cell potentials. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. . Standard Reduction Potential Sn4+.
From www.bartleby.com
Answered Consider the following redox reaction… bartleby Standard Reduction Potential Sn4+ describe and relate the definitions of electrode and cell potentials. standard reduction potential (e°): assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Standard reduction potentials by value. Standard. Standard Reduction Potential Sn4+.
From testbook.com
Reduction Potential Measurement, Half Cells, Differences & More Standard Reduction Potential Sn4+ standard reduction potential (e°): identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Standard reduction potentials by value. 1 m for solutes) usually at 298.15 k;.. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED The standard reduction potential for the halfreaction Sn4 Standard Reduction Potential Sn4+ Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 m for solutes) usually at 298.15 k;. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Standard reduction potentials by value. describe and relate the definitions of electrode and cell potentials. standard reduction potential (e°): a cell constructed by. Standard Reduction Potential Sn4+.
From homeworkocean.com
(Solved Homework) Standard reduction potentials Use the table of Standard Reduction Potential Sn4+ Interpret electrode potentials in terms of relative oxidant and reductant strengths. The value of the reduction under standard conditions (1 bar or 1 atm for gases; assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. The following table provides eo for selected reduction. Standard reduction potentials by value. 1 m for. Standard Reduction Potential Sn4+.
From edurev.in
Standard electrode potential for sn4+/sn2+couple is +0.15 V and that Standard Reduction Potential Sn4+ Interpret electrode potentials in terms of relative oxidant and reductant strengths. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Standard reduction potentials by value. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. describe and relate the definitions. Standard Reduction Potential Sn4+.
From www.toppr.com
Standard potential (E) some halfreactions are given below Sn4+ + 2e → Standard Reduction Potential Sn4+ describe and relate the definitions of electrode and cell potentials. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The following table provides eo for selected reduction. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. Interpret electrode potentials in terms of relative oxidant and reductant strengths. assigning. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED The standard reduction potential for the halfreaction Sn4 Standard Reduction Potential Sn4+ 1 m for solutes) usually at 298.15 k;. describe and relate the definitions of electrode and cell potentials. Standard reduction potentials by value. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Standard reduction potentials by value. identify how to view standard reduction potentials from the perspective. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Selective Oxidation The standard reduction potential for the Standard Reduction Potential Sn4+ The value of the reduction under standard conditions (1 bar or 1 atm for gases; 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. The following table provides eo for selected reduction. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. assigning the potential of the standard hydrogen. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED The standard reduction potential of (Sn4+ + 2e > Sn2+ ) is 0. Standard Reduction Potential Sn4+ Standard reduction potentials by value. The following table provides eo for selected reduction. standard reduction potential (e°): Standard reduction potentials by value. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Selective Oxidation The standard reduction potential for the Standard Reduction Potential Sn4+ Interpret electrode potentials in terms of relative oxidant and reductant strengths. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. The following table provides eo for selected reduction. a cell constructed by coupling a. Standard Reduction Potential Sn4+.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Sn4+ The following table provides eo for selected reduction. describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. standard reduction potential (e°): a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Standard reduction potentials by value.. Standard Reduction Potential Sn4+.
From chem.libretexts.org
17.3 Standard Reduction Potentials Chemistry LibreTexts Standard Reduction Potential Sn4+ Standard reduction potentials by value. The following table provides eo for selected reduction. 1 m for solutes) usually at 298.15 k;. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. The. Standard Reduction Potential Sn4+.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Sn4+ Standard reduction potentials by value. describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. standard reduction potential (e°): a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. 1 m for solutes) usually at 298.15 k;.. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Selective Oxidation The standard reduction potential for the Standard Reduction Potential Sn4+ a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Interpret electrode potentials in terms of relative oxidant and reductant strengths. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. 1 m for solutes) usually at 298.15 k;. identify how. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Standard reduction potentials Reduction halfreaction Ag(aq Standard Reduction Potential Sn4+ The following table provides eo for selected reduction. standard reduction potential (e°): Standard reduction potentials by value. Standard reduction potentials by value. The value of the reduction under standard conditions (1 bar or 1 atm for gases; 1 m for solutes) usually at 298.15 k;. Interpret electrode potentials in terms of relative oxidant and reductant strengths. a cell. Standard Reduction Potential Sn4+.
From ch302.cm.utexas.edu
stdpotsshortlist.png Standard Reduction Potential Sn4+ a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Interpret electrode potentials in terms of relative oxidant and reductant strengths. describe and relate the definitions of electrode and cell potentials. The value of the reduction under standard conditions (1 bar or 1 atm for gases; identify how. Standard Reduction Potential Sn4+.
From www.chegg.com
Solved Questions 33 and 34 uses the following standard Standard Reduction Potential Sn4+ Standard reduction potentials by value. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Standard reduction potentials by value. Interpret electrode potentials in terms of relative oxidant and reductant strengths. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. . Standard Reduction Potential Sn4+.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Sn4+ assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. describe and relate the definitions of electrode and cell potentials. 1 m for solutes) usually at 298.15 k;. standard reduction potential (e°): Interpret electrode potentials in terms of relative oxidant and reductant strengths. Standard reduction potentials by value. The value. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Using the following standard reduction potentials, Sn4+(aq Standard Reduction Potential Sn4+ assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Standard reduction potentials by value. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Interpret. Standard Reduction Potential Sn4+.
From www.toppr.com
The standard reduction potential at 25^o C for the following half Standard Reduction Potential Sn4+ describe and relate the definitions of electrode and cell potentials. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. Interpret electrode potentials in terms of relative oxidant and reductant strengths.. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Selective Reduction The standard reduction potential for the Standard Reduction Potential Sn4+ Standard reduction potentials by value. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 m for solutes) usually at 298.15 k;. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. a. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED The standard reduction potential for the halfreaction Sn4 Standard Reduction Potential Sn4+ Standard reduction potentials by value. describe and relate the definitions of electrode and cell potentials. standard reduction potential (e°): a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Interpret electrode potentials in. Standard Reduction Potential Sn4+.
From www.flinnsci.ca
Standard Reduction Potential Chart Flinn Scientific Standard Reduction Potential Sn4+ 1 m for solutes) usually at 298.15 k;. identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. Standard reduction potentials by value. Interpret electrode potentials in terms of relative oxidant and reductant strengths. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts.. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Selective Reduction The standard reduction potential for the Standard Reduction Potential Sn4+ Interpret electrode potentials in terms of relative oxidant and reductant strengths. a cell constructed by coupling a standard copper electrode and a standard magnesium electrode has emf of 2.7 volts. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Standard reduction potentials by value. standard reduction potential (e°): Standard. Standard Reduction Potential Sn4+.
From www.numerade.com
SOLVED Given the following cell notation and list of standard Standard Reduction Potential Sn4+ describe and relate the definitions of electrode and cell potentials. Standard reduction potentials by value. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Standard reduction potentials by value. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The following table provides eo for selected reduction. assigning the potential of. Standard Reduction Potential Sn4+.
From pubs.acs.org
Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Standard Reduction Potential Sn4+ identify how to view standard reduction potentials from the perspective of viable reducing and oxidizing. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. The following table provides eo for selected reduction. describe and relate the. Standard Reduction Potential Sn4+.