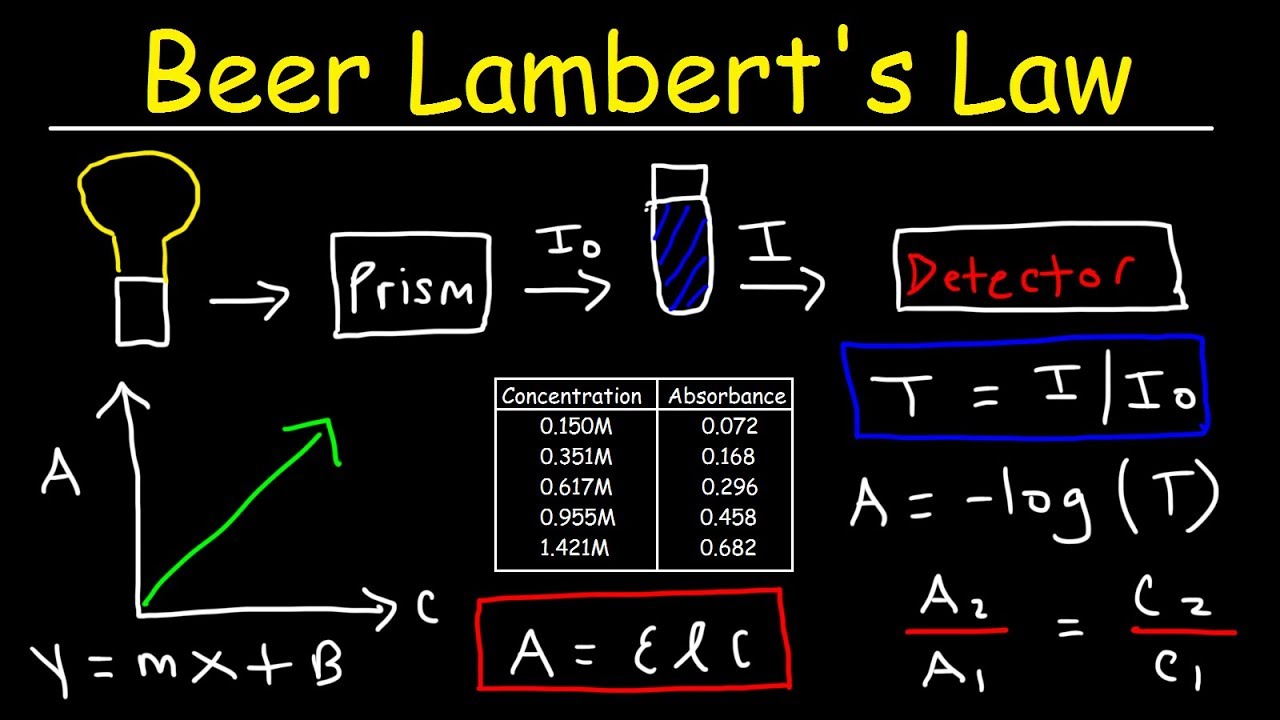
Beer's Law Lab Conclusion . Use the straight line fit on. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. His law was in full effect because we saw absorbance rise as. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. This relationship is called the. Determine order of reaction by measuring the concentration of. A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Beer’s law fabrizio chigne valerga lab partner:
from www.youtube.com
Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Use the straight line fit on. His law was in full effect because we saw absorbance rise as. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. Beer’s law fabrizio chigne valerga lab partner: Determine order of reaction by measuring the concentration of.
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry
Beer's Law Lab Conclusion This relationship is called the. Beer’s law fabrizio chigne valerga lab partner: Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Use the straight line fit on. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. His law was in full effect because we saw absorbance rise as. Determine order of reaction by measuring the concentration of. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Lab Conclusion His law was in full effect because we saw absorbance rise as. Use the straight line fit on. This relationship is called the. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Calculate the concentration. Beer's Law Lab Conclusion.
From mckennaroscervantes.blogspot.com
Beer's Lambert Law Equation MckennarosCervantes Beer's Law Lab Conclusion Use the straight line fit on. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Determine order of reaction by measuring the concentration of. His law was in full effect because we saw absorbance rise as. Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. The amount of light. Beer's Law Lab Conclusion.
From www.studocu.com
Beers law experiment 3 3 EXPERIMENT 3 BEER’S LAW OBJECTIVES • To Beer's Law Lab Conclusion This relationship is called the. Use the straight line fit on. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Since the concentration, path length and molar absorptivity are all. Beer's Law Lab Conclusion.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Lab Conclusion Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. Beer’s law fabrizio chigne valerga lab partner: Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. His law was in full effect because we saw absorbance rise as. As beer’s law states, absorption is. Beer's Law Lab Conclusion.
From www.studocu.com
Beer’s Law PreLab Worksheet Complete the worksheet and submit it to Beer's Law Lab Conclusion Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Beer’s law fabrizio chigne valerga lab partner: The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer's Law Lab Conclusion.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Lab Conclusion The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Since the concentration, path length and. Beer's Law Lab Conclusion.
From www.studocu.com
Lab 4 Beer's Law Lab 4 Beer’s Law OBJECTIVES. The purpose of this Beer's Law Lab Conclusion Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. Beer’s law fabrizio chigne valerga lab partner: The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. His law was in full effect because we saw absorbance rise as. Reference source not. Beer's Law Lab Conclusion.
From www.chegg.com
Spectrophotometry and Beer's Law spermanent Lab Beer's Law Lab Conclusion The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). This relationship is called the. Since the concentration, path length. Beer's Law Lab Conclusion.
From www.learnatnoon.com
What are some Limitations of Beer Lambert Law? Noon Academy Beer's Law Lab Conclusion This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A colorimeter is used to determine how much. Beer's Law Lab Conclusion.
From studylib.net
Beer's Law Lab Beer's Law Lab Conclusion Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. His law was in full effect because we saw absorbance rise as. Use the straight line fit on. This relationship is called the. Conclusion in. Beer's Law Lab Conclusion.
From www.studocu.com
Chem 181 4 Beer's Law Fall 2020 Determining the Concentration of a Beer's Law Lab Conclusion This relationship is called the. Determine order of reaction by measuring the concentration of. Use the straight line fit on. Beer’s law fabrizio chigne valerga lab partner: Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that a species absorbs. Beer's Law Lab Conclusion.
From www.studocu.com
Chem 227 Beer's Law Lap report of beer lab Abstract Using a Beer's Law Lab Conclusion Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Determine order of reaction by measuring the concentration of. His law was in full effect because we saw absorbance rise as. Beer’s law fabrizio chigne valerga lab partner: Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law Lab Conclusion.
From www.softpedia.com
Download Beer's Law Lab Beer's Law Lab Conclusion This relationship is called the. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Conclusion in this lab, we determined the concentration of an unknown. Beer's Law Lab Conclusion.
From www.studocu.com
Beers Law Lab Lab Report from General Chemistry 2 Lab 50/50 score Beer's Law Lab Conclusion His law was in full effect because we saw absorbance rise as. Use the straight line fit on. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. Reference source not found) shows a straight line equation when the. Beer's Law Lab Conclusion.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Beer's Law Lab Conclusion His law was in full effect because we saw absorbance rise as. Use the straight line fit on. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Beer’s law fabrizio chigne valerga lab partner: Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we. Beer's Law Lab Conclusion.
From studylib.net
Beer's Law Lab Beer's Law Lab Conclusion Use the straight line fit on. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. His law was in full effect because we saw absorbance rise as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer's Law Lab Conclusion.
From studylib.net
Beer's Law Lab Beer's Law Lab Conclusion Beer’s law fabrizio chigne valerga lab partner: Use the straight line fit on. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since. Beer's Law Lab Conclusion.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Beer's Law Lab Conclusion Determine order of reaction by measuring the concentration of. Use the straight line fit on. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. Beer’s law fabrizio chigne valerga lab partner: His law was in full effect because we saw. Beer's Law Lab Conclusion.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Lab Conclusion This relationship is called the. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Since the concentration, path length and molar absorptivity are all. Beer's Law Lab Conclusion.
From www.studocu.com
Beer's Law Lab Report Name Darius Savoy Partner Sophie Hudson Beer's Law Lab Conclusion A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you. Beer's Law Lab Conclusion.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Lab Conclusion Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. This relationship is called the. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. Determine order of reaction by measuring the concentration of. Beer’s law fabrizio chigne valerga lab partner: His law was in full. Beer's Law Lab Conclusion.
From www.scribd.com
Beer S Law Lab Instructions PDF Absorbance Molar Concentration Beer's Law Lab Conclusion This relationship is called the. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). His law was in full effect because we saw absorbance rise as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that. Beer's Law Lab Conclusion.
From www.studocu.com
Lab report Experiment 2. Spectroscopic Analysis using Beer’s Law Beer's Law Lab Conclusion Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. Use the straight line fit on. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). This relationship is. Beer's Law Lab Conclusion.
From www.studocu.com
Beer's Law Lab Report Name Pariya Fard Saberi Partner Stefania Beer's Law Lab Conclusion As beer’s law states, absorption is measured upon the amount of light a compound absorbs. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Beer’s law fabrizio chigne valerga lab. Beer's Law Lab Conclusion.
From www.chegg.com
Solved BEERS LAWTransmi COLORIMETRYBEER'S LAW LAB REPORT Beer's Law Lab Conclusion Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). This relationship is called the. Determine order of reaction by measuring the concentration of. The amount of light that a species absorbs. Beer's Law Lab Conclusion.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Lab Conclusion A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Use the straight line fit on. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Determine order of reaction by measuring the concentration of. Beer’s law fabrizio chigne valerga lab partner: Reference source not found) shows a straight line. Beer's Law Lab Conclusion.
From www.studocu.com
Beer's Law Beer's Law Lab Fall Semester Grade A+ Name Partner Beer's Law Lab Conclusion His law was in full effect because we saw absorbance rise as. A colorimeter is used to determine how much light a solution absorbs, and then produces the number. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that a. Beer's Law Lab Conclusion.
From www.slideserve.com
PPT Chem. 133 3/18 Lecture PowerPoint Presentation, free download Beer's Law Lab Conclusion Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Determine order of reaction by measuring the concentration of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively. Beer's Law Lab Conclusion.
From www.youtube.com
Experiment 13 Beer's Law YouTube Beer's Law Lab Conclusion Beer’s law fabrizio chigne valerga lab partner: Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. His law was in full effect because we saw absorbance rise as. Determine order of reaction by measuring the concentration of. A colorimeter is used to determine how. Beer's Law Lab Conclusion.
From www.slideserve.com
PPT beers law lab experiment PowerPoint Presentation, free download Beer's Law Lab Conclusion A colorimeter is used to determine how much light a solution absorbs, and then produces the number. His law was in full effect because we saw absorbance rise as. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer's Law Lab Conclusion.
From www.studocu.com
CHEM 112 Experiment 4 Beer's Law Lab Report Chem 112 queensu Beer's Law Lab Conclusion Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Determine order of reaction by measuring the concentration of. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional. Beer's Law Lab Conclusion.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Lab Conclusion Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer's Law Lab Conclusion.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Lab Conclusion His law was in full effect because we saw absorbance rise as. Determine order of reaction by measuring the concentration of. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Conclusion in this lab, we determined the concentration of an unknown copper sulfate using beer’s law. Since the concentration, path. Beer's Law Lab Conclusion.
From www.studocu.com
Beer's Law Experiment Experiment 4 Beer’s Law. Purpose The purpose Beer's Law Lab Conclusion Use the straight line fit on. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. Determine order of reaction by measuring the concentration of. Reference source not found) shows a straight line equation when the absorbance versus concentration is plotted, as you did. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy. Beer's Law Lab Conclusion.
From chemistrysources.com
قانون بيير (بير) Beer’s Law التعريف و المعادلة مصادر الكيمياء Beer's Law Lab Conclusion Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. As beer’s law states, absorption is measured upon the amount of light a compound absorbs. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related. Beer's Law Lab Conclusion.