Ionic Crystalline Solid Properties . What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic solids are a form of crystalline solid.
from www.chegg.com
An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids are a form of crystalline solid. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong.
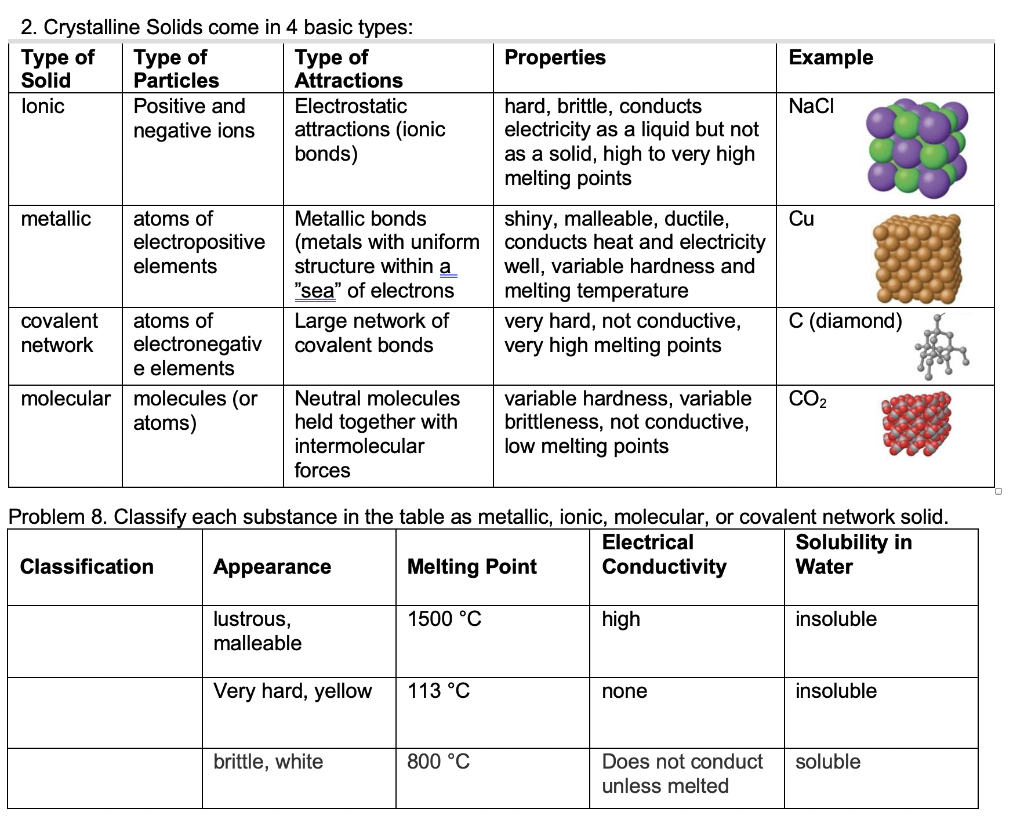
Solved Properties Example 2. Crystalline Solids come in 4
Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic solids are a form of crystalline solid. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely.
From schoolbag.info
FIGURE 12.1 Classifications of solids according to predominant bonding Ionic Crystalline Solid Properties What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic solids, such as sodium. Ionic Crystalline Solid Properties.
From www.slideshare.net
Ionic solids Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic. Ionic Crystalline Solid Properties.
From www.ck12.org
Comparing Crystals Overview ( Video ) Chemistry CK12 Foundation Ionic Crystalline Solid Properties Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. An ionic solid is made up of. Ionic Crystalline Solid Properties.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Ionic Crystalline Solid Properties What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are. Ionic Crystalline Solid Properties.
From www.chegg.com
Solved Properties Example 2. Crystalline Solids come in 4 Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged. Ionic Crystalline Solid Properties.
From chem.libretexts.org
6.2 Comparing Ionic and Molecular Substances Chemistry LibreTexts Ionic Crystalline Solid Properties Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held. Ionic Crystalline Solid Properties.
From eduinput.com
Crystalline solids Properties, types, examples use Ionic Crystalline Solid Properties Ionic solids are a form of crystalline solid. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions. Ionic Crystalline Solid Properties.
From psiberg.com
Types of Crystalline Solids The Seven Crystal System Ionic Crystalline Solid Properties Ionic solids are a form of crystalline solid. An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? In nature, the ordered arrangement of ionic solids gives rise. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Ionic and covalent Bonding and Properties of Solids PowerPoint Ionic Crystalline Solid Properties What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Ionic Bonding Part I PowerPoint Presentation, free download ID Ionic Crystalline Solid Properties Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic solids are those crystalline solids in which the. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Chapter 8 Chemical Bonding PowerPoint Presentation, free Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction. Ionic Crystalline Solid Properties.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged. Ionic Crystalline Solid Properties.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Ionic Crystalline Solid Properties Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids are a form of crystalline. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation Ionic Crystalline Solid Properties What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids are those crystalline. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Unit 2 Molecular and Ionic Compound Structure and Properties Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. What is an ionic solid, what are its typical physical properties, and what kinds of elements. Ionic Crystalline Solid Properties.
From slideplayer.com
Properties of Ionic Compounds ppt download Ionic Crystalline Solid Properties In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic solids are a form of crystalline solid. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids are. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download Ionic Crystalline Solid Properties What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. An. Ionic Crystalline Solid Properties.
From www.learnatnoon.com
What are the Properties of Crystalline Solids? Noon Academy Ionic Crystalline Solid Properties In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. Ionic solids are those crystalline solids in which the. Ionic Crystalline Solid Properties.
From www.w3schools.blog
Ionic Solids W3schools Ionic Crystalline Solid Properties Ionic solids are a form of crystalline solid. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids, such as sodium chloride and nickel oxide, are. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID7049909 Ionic Crystalline Solid Properties In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic solids are a form of crystalline. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Properties of Solids PowerPoint Presentation, free download ID Ionic Crystalline Solid Properties What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic solids are a form of crystalline solid. An ionic solid is made up of cations (positive ions) and anions (negative ions) held together. Ionic Crystalline Solid Properties.
From www.numerade.com
SOLVED Ln molecular solid amorphous solid crystalline metallic solid Ionic Crystalline Solid Properties Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. What is an ionic solid, what are. Ionic Crystalline Solid Properties.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts Ionic Crystalline Solid Properties Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic solids are a form of crystalline solid. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Structure & Properties of Solids PowerPoint Presentation ID1990451 Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one. Ionic Crystalline Solid Properties.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction. Ionic Crystalline Solid Properties.
From slideplayer.com
Solid state Chemistry (CHEM 422) ppt download Ionic Crystalline Solid Properties What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic. Ionic Crystalline Solid Properties.
From www.pinterest.com
PPT Crystalline Solids PowerPoint Presentation, free download ID Ionic Crystalline Solid Properties Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids are a form of crystalline solid. In nature, the ordered arrangement of ionic solids gives rise to beautiful. Ionic Crystalline Solid Properties.
From sciencenotes.org
Ionic Compound Properties Ionic Crystalline Solid Properties In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. Ionic solids are a form of crystalline. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Properties of Solids PowerPoint Presentation, free download ID Ionic Crystalline Solid Properties Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic. Ionic Crystalline Solid Properties.
From animalia-life.club
Covalent Network Solids Ionic Crystalline Solid Properties Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. What is an ionic solid, what are. Ionic Crystalline Solid Properties.
From studylib.net
Metallic and Ionic Solids Types of Solids Properties of Solids Crystal Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by. What is an ionic solid, what are its typical physical properties, and what kinds of elements. Ionic Crystalline Solid Properties.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Ionic Crystalline Solid Properties Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together. Ionic Crystalline Solid Properties.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Crystalline Solid Properties Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held together by strong. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I PART 2 PowerPoint Presentation Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. What is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are. Ionic Crystalline Solid Properties.
From www.slideserve.com
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation Ionic Crystalline Solid Properties An ionic solid is made up of cations (positive ions) and anions (negative ions) held together by an electrostatic attraction between the oppositely. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. Ionic solids are those crystalline solids in which the particles forming the crystal are positively and negatively charged ions, held. Ionic Crystalline Solid Properties.