Medical Device Regulations Fda . the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. learn about fda's role, authority, and process for regulating medical devices in the u.s. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This presentation covers device definition,. Fda regulates the sale of medical device products (including diagnostic tests). learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. fda's role in regulating medical devices. Manufacturers must establish and follow quality systems to help ensure that their products consistently meet.
from www.youtube.com
the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. This presentation covers device definition,. learn about fda's role, authority, and process for regulating medical devices in the u.s. fda's role in regulating medical devices. Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. Fda regulates the sale of medical device products (including diagnostic tests). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr).
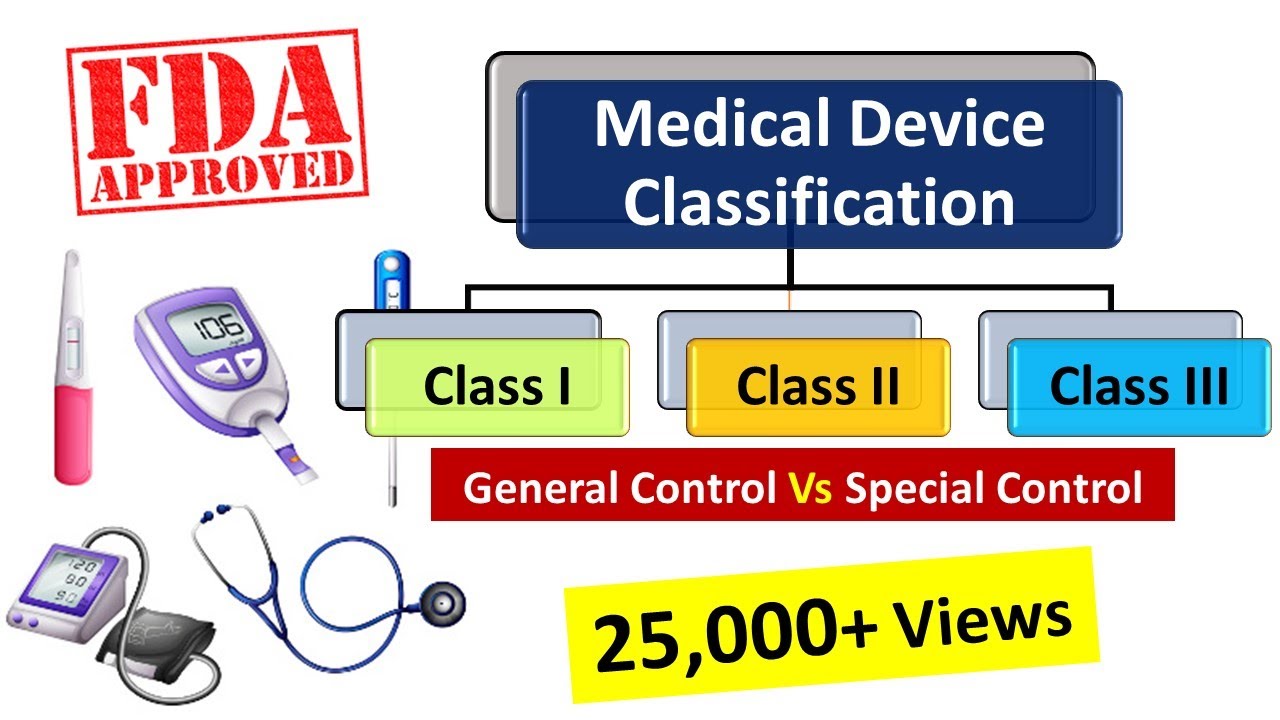
Medical Devices classification as per FDA Medical Device Regulations
Medical Device Regulations Fda Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. This presentation covers device definition,. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. fda's role in regulating medical devices. Fda regulates the sale of medical device products (including diagnostic tests). learn about fda's role, authority, and process for regulating medical devices in the u.s. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Regulations Fda Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. fda's role in regulating medical devices. Fda regulates the sale of medical device products (including diagnostic tests). learn about fda's role, authority, and process for regulating medical devices in the u.s. This presentation covers device definition,. the code of federal regulations (cfr). Medical Device Regulations Fda.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Regulations Fda Fda regulates the sale of medical device products (including diagnostic tests). This presentation covers device definition,. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. labeling regulations pertaining. Medical Device Regulations Fda.
From www.quantib.com
A 101 guide to the FDA regulatory process for AI radiology software Medical Device Regulations Fda fda's role in regulating medical devices. Fda regulates the sale of medical device products (including diagnostic tests). learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. This presentation covers device definition,. the code of federal regulations (cfr) is the official legal print publication containing the codification of the. Medical Device Regulations Fda.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulations Fda the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. Fda regulates the sale of medical device products (including diagnostic tests). This presentation covers device definition,. Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. labeling regulations pertaining to medical devices are. Medical Device Regulations Fda.
From www.slideshare.net
Overview of FDA Regulation of Medical Devices Medical Device Regulations Fda fda's role in regulating medical devices. learn about fda's role, authority, and process for regulating medical devices in the u.s. Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. Fda regulates the sale of medical device products (including diagnostic tests). the code of federal regulations (cfr) is the official legal print. Medical Device Regulations Fda.
From www.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Regulations Fda fda's role in regulating medical devices. the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. learn about fda's role, authority, and process for regulating medical devices in the u.s. This presentation covers device definition,. Fda regulates the sale of medical device products (including diagnostic tests). Manufacturers. Medical Device Regulations Fda.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Medical Device Regulations Fda Fda regulates the sale of medical device products (including diagnostic tests). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. This presentation covers device definition,. the. Medical Device Regulations Fda.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Medical Device Regulations Fda Fda regulates the sale of medical device products (including diagnostic tests). learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. This presentation covers device definition,. the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. learn about fda's. Medical Device Regulations Fda.
From www.cell.com
The Regulation of Wearable Medical Devices Trends in Biotechnology Medical Device Regulations Fda This presentation covers device definition,. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. the code of federal regulations (cfr) is the official legal print publication containing the codification of. Medical Device Regulations Fda.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Medical Device Regulations Fda learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. learn about fda's role, authority, and process for regulating medical devices in the u.s. Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. fda's role in regulating medical devices. Fda regulates the sale. Medical Device Regulations Fda.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Device Regulations Fda fda's role in regulating medical devices. Fda regulates the sale of medical device products (including diagnostic tests). This presentation covers device definition,. learn about fda's role, authority, and process for regulating medical devices in the u.s. the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. Manufacturers. Medical Device Regulations Fda.
From www.youtube.com
Medical Device Regulations / FDA Approval YouTube Medical Device Regulations Fda Fda regulates the sale of medical device products (including diagnostic tests). fda's role in regulating medical devices. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This presentation covers device definition,. learn about the fda's role in regulating medical devices in the u.s., including. Medical Device Regulations Fda.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulations Fda This presentation covers device definition,. fda's role in regulating medical devices. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. Fda regulates the sale of medical device products (including diagnostic tests). learn about fda's role, authority, and process for regulating medical devices in the u.s. Manufacturers must establish. Medical Device Regulations Fda.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Regulations Fda fda's role in regulating medical devices. Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This presentation covers device definition,. learn about the fda's role in regulating medical devices. Medical Device Regulations Fda.
From blog.sierralabs.com
6 Regulatory Pathways to Bring Your Medical Device to Market Medical Device Regulations Fda labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. fda's role in regulating medical devices. This presentation covers device definition,. Manufacturers must establish and follow quality. Medical Device Regulations Fda.
From www.slideshare.net
FDA regulation for medical devices Medical Device Regulations Fda learn about fda's role, authority, and process for regulating medical devices in the u.s. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This presentation covers device definition,. fda's role in regulating medical devices. the code of federal regulations (cfr) is the official. Medical Device Regulations Fda.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Regulations Fda learn about fda's role, authority, and process for regulating medical devices in the u.s. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. Manufacturers must establish. Medical Device Regulations Fda.
From www.presentationeze.com
FDA Regulatory Requirements Medical Devices.PresentationEZE Medical Device Regulations Fda labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. Fda regulates the sale of medical device products (including diagnostic tests). fda's role in regulating medical devices. the code of. Medical Device Regulations Fda.
From www.protoexpress.com
Medical Device Regulations for PCBA Sierra Circuits Medical Device Regulations Fda Fda regulates the sale of medical device products (including diagnostic tests). Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. the code of federal regulations (cfr) is the official legal print publication containing the. Medical Device Regulations Fda.
From www.slideshare.net
Overview of FDA Regulation of Medical Devices Medical Device Regulations Fda Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. Fda regulates the sale of medical device products (including diagnostic tests). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). learn about fda's role, authority, and process for regulating medical. Medical Device Regulations Fda.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Device Regulations Fda the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. Fda regulates the sale of medical device products (including diagnostic tests). learn about fda's role, authority, and process for. Medical Device Regulations Fda.
From www.youtube.com
FDA Regulation of Medical Devices and Software/Apps YouTube Medical Device Regulations Fda This presentation covers device definition,. the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. Fda regulates the sale of medical device products (including diagnostic tests). Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. learn about the fda's role in regulating. Medical Device Regulations Fda.
From www.slideshare.net
US FDA medical device approval chart Emergo Group Medical Device Regulations Fda Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This presentation covers device definition,. learn about fda's role, authority, and process for regulating medical devices in the u.s. learn. Medical Device Regulations Fda.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Regulations Fda fda's role in regulating medical devices. Fda regulates the sale of medical device products (including diagnostic tests). This presentation covers device definition,. Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Medical Device Regulations Fda.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Regulations Fda labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. fda's role in regulating medical devices. the code of federal regulations (cfr) is the official legal. Medical Device Regulations Fda.
From www.slideserve.com
PPT Overview of FDA Device Regulations PowerPoint Presentation, free Medical Device Regulations Fda the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. learn about fda's role, authority, and process for regulating medical devices in the u.s. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This. Medical Device Regulations Fda.
From www.presentationeze.com
FDA medical device classification PresentationEZE Medical Device Regulations Fda the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. Fda regulates the sale of medical device products (including diagnostic tests). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This presentation covers device definition,.. Medical Device Regulations Fda.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Regulations Fda the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). fda's role in regulating medical devices. learn about the fda's role in regulating medical. Medical Device Regulations Fda.
From phs.weill.cornell.edu
Food and Drug Administration Surveillance and Medical Device Regulation Medical Device Regulations Fda This presentation covers device definition,. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. Fda regulates the sale of medical device products (including diagnostic tests). Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. the code of federal regulations (cfr) is the official. Medical Device Regulations Fda.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Medical Device Regulations Fda Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. fda's role in regulating medical devices. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Fda regulates the sale of medical device products (including diagnostic tests). learn about the. Medical Device Regulations Fda.
From www.presentationeze.com
Medical Device Regulations. Design Requirements PresentationEZE Medical Device Regulations Fda labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. learn about fda's role, authority, and process for regulating medical devices in the u.s. fda's role in regulating medical devices.. Medical Device Regulations Fda.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulations Fda Fda regulates the sale of medical device products (including diagnostic tests). This presentation covers device definition,. the code of federal regulations (cfr) is the official legal print publication containing the codification of the general and permanent. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. Manufacturers must establish and. Medical Device Regulations Fda.
From sterlingmedicaldevices.com
FDA Medical Device Regulation Guidance for 2022 Medical Device Regulations Fda Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. This presentation covers device definition,. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. fda's role in regulating medical devices. learn about fda's role, authority, and process for regulating medical devices in the. Medical Device Regulations Fda.
From www.acuity.com
An Update on FDA Regulations Acuity Medical Device Regulations Fda learn about fda's role, authority, and process for regulating medical devices in the u.s. This presentation covers device definition,. Fda regulates the sale of medical device products (including diagnostic tests). Manufacturers must establish and follow quality systems to help ensure that their products consistently meet. the code of federal regulations (cfr) is the official legal print publication containing. Medical Device Regulations Fda.
From www.slideserve.com
PPT Preparing for an IDE Application PowerPoint Presentation, free Medical Device Regulations Fda fda's role in regulating medical devices. learn about fda's role, authority, and process for regulating medical devices in the u.s. learn about the fda's role in regulating medical devices in the u.s., including approvals, clearances, recalls, and safety. the code of federal regulations (cfr) is the official legal print publication containing the codification of the general. Medical Device Regulations Fda.