Application Of Calorimeter . a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Ac (alternating current) calorimeter 194f, 217 accelerating. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from sites.psu.edu
Ac (alternating current) calorimeter 194f, 217 accelerating. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
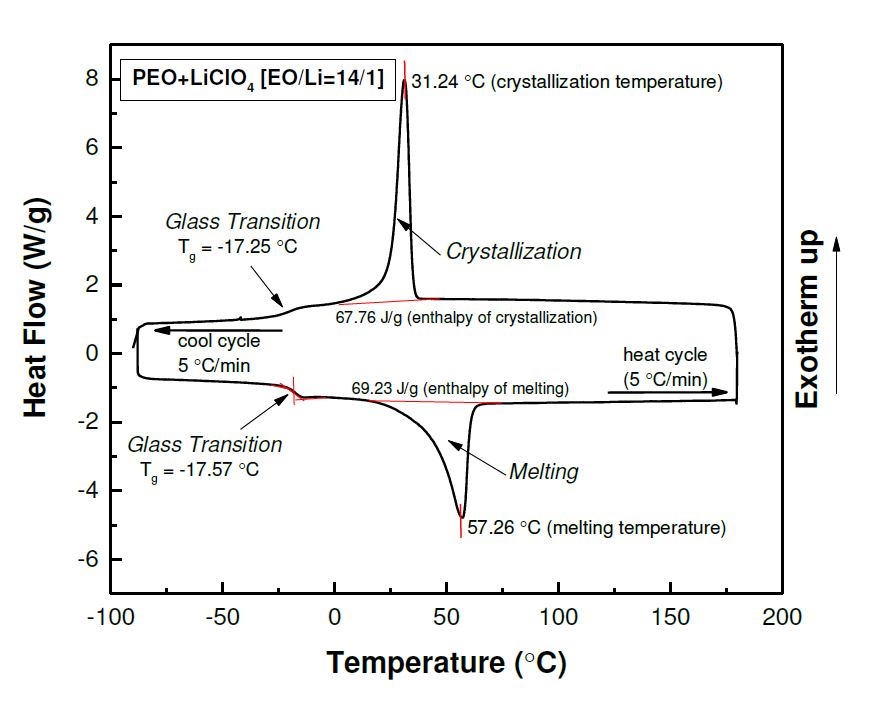
Differential Scanning Calorimetry Janna Maranas Research Group
Application Of Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Ac (alternating current) calorimeter 194f, 217 accelerating. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical.
From www.researchgate.net
28 Schematic diagram of Differential scanning calorimetry. Download Application Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. Ac (alternating. Application Of Calorimeter.
From drivenheisenberg.blogspot.com
Which Diagram Is A Bomb Calorimeter Drivenheisenberg Application Of Calorimeter apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. Compare heat flow from hot to cold objects in an. Application Of Calorimeter.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Application Of Calorimeter calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a. Application Of Calorimeter.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Application Of Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a. Application Of Calorimeter.
From www.youtube.com
Coffee Cup Calorimetry YouTube Application Of Calorimeter apply the first law of thermodynamics to calorimetry. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. Ac (alternating current) calorimeter 194f, 217 accelerating. . Application Of Calorimeter.
From pathwaystochemistry.com
Calorimetry Pathways to Chemistry Application Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Ac (alternating current) calorimeter 194f, 217 accelerating. a calorimeter is a device. Application Of Calorimeter.
From novapublishers.com
Differential Scanning Calorimetry Basics and Applications Nova Application Of Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. Application Of Calorimeter.
From testbook.com
Principle of Calorimetry Definition, Formula, Uses, Examples Application Of Calorimeter Ac (alternating current) calorimeter 194f, 217 accelerating. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or. Application Of Calorimeter.
From www.tainstruments.com
Differential Scanning Calorimeter DSC2500 (includes auto sampler, MDSC Application Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimeter, device. Application Of Calorimeter.
From www.walmart.com
Electric Calorimeter Application Of Calorimeter a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a. Application Of Calorimeter.
From www.youtube.com
050 Calorimetry YouTube Application Of Calorimeter a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the. Application Of Calorimeter.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Application Of Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Application Of Calorimeter.
From university.pressbooks.pub
10.2 Calorimetry Chemistry Fundamentals Application Of Calorimeter apply the first law of thermodynamics to calorimetry. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Compare heat flow from hot to cold. Application Of Calorimeter.
From schoolbag.info
14 Thermodynamics STEP 4 Review the Knowledge You Need to Score High Application Of Calorimeter apply the first law of thermodynamics to calorimetry. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat. Application Of Calorimeter.
From www.scribd.com
B. Heat Capacity of Calorimeter PDF Application Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Ac (alternating current) calorimeter 194f, 217 accelerating. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. apply the first law of thermodynamics to calorimetry. calorimetry is a branch of science. Application Of Calorimeter.
From www.scribd.com
Differential Scanning Calorimetry (DSC) Differential Scanning Application Of Calorimeter a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Application Of Calorimeter.
From profilab24.com
IKA Calorimeter C 6000 isoperibol Package 2/12, without chiller Application Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Ac (alternating current) calorimeter 194f, 217 accelerating. a calorimeter is a device used for calorimetry, or the process of measuring the heat of. Application Of Calorimeter.
From www.indiamart.com
Bomb Calorimeter with Automatic Calculation, For Coal And Briquette Application Of Calorimeter Ac (alternating current) calorimeter 194f, 217 accelerating. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Compare heat flow from hot to cold objects in an ideal. Application Of Calorimeter.
From www.degruyter.com
Application of Calorimetry in Life Sciences Application Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the. Application Of Calorimeter.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Application Of Calorimeter apply the first law of thermodynamics to calorimetry. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. Ac (alternating current) calorimeter 194f, 217 accelerating. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters. Application Of Calorimeter.
From www.youtube.com
Differential Scanning Calorimetry DSC Principle Instrumentation Application Of Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimetry is. Application Of Calorimeter.
From www.indiamart.com
Digital Bomb Calorimeter Model CC01/M2, डिजिटल बम कैलोरीमीटर Application Of Calorimeter apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters is an important chemistry lab instrument devices that measure the amount of heat. Application Of Calorimeter.
From sites.psu.edu
Differential Scanning Calorimetry Janna Maranas Research Group Application Of Calorimeter Ac (alternating current) calorimeter 194f, 217 accelerating. apply the first law of thermodynamics to calorimetry. calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter,. Application Of Calorimeter.
From phdsciencegyan.com
Maharashtra Board Class 8 Science Solutions Chapter 14 Measurement and Application Of Calorimeter Ac (alternating current) calorimeter 194f, 217 accelerating. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimetry is a branch of science concerned. Application Of Calorimeter.
From www.tainstruments.com
Differential Scanning Calorimeter DSC2500 (includes auto sampler and Application Of Calorimeter calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Ac (alternating current) calorimeter 194f, 217 accelerating. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is a branch of science concerned with measuring. Application Of Calorimeter.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Application Of Calorimeter a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. apply the first law of thermodynamics to calorimetry. Ac (alternating current) calorimeter 194f, 217 accelerating. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is. Application Of Calorimeter.
From www.mdpi.com
Polymers Free FullText Application of Differential Scanning Application Of Calorimeter a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter. Application Of Calorimeter.
From www.mdpi.com
Polymers Free FullText Application of Differential Scanning Application Of Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. Ac (alternating current) calorimeter 194f, 217 accelerating. a calorimeter is a device used for calorimetry, or the. Application Of Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Application Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is. Application Of Calorimeter.
From www.tainstruments.com
Differential Scanning Calorimeter DSC 2500 (includes auto sampler, MDSC Application Of Calorimeter Ac (alternating current) calorimeter 194f, 217 accelerating. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction. Application Of Calorimeter.
From ar.inspiredpencil.com
Basic Calorimeter Application Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a. Application Of Calorimeter.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The Application Of Calorimeter calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. Ac (alternating current) calorimeter 194f, 217 accelerating. calorimeters is an important chemistry lab instrument devices that. Application Of Calorimeter.
From scientificservices.eu
Differential Scanning Calorimeter UseScience Application Of Calorimeter a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. . Application Of Calorimeter.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Application Of Calorimeter a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Application Of Calorimeter.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Application Of Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the. Application Of Calorimeter.