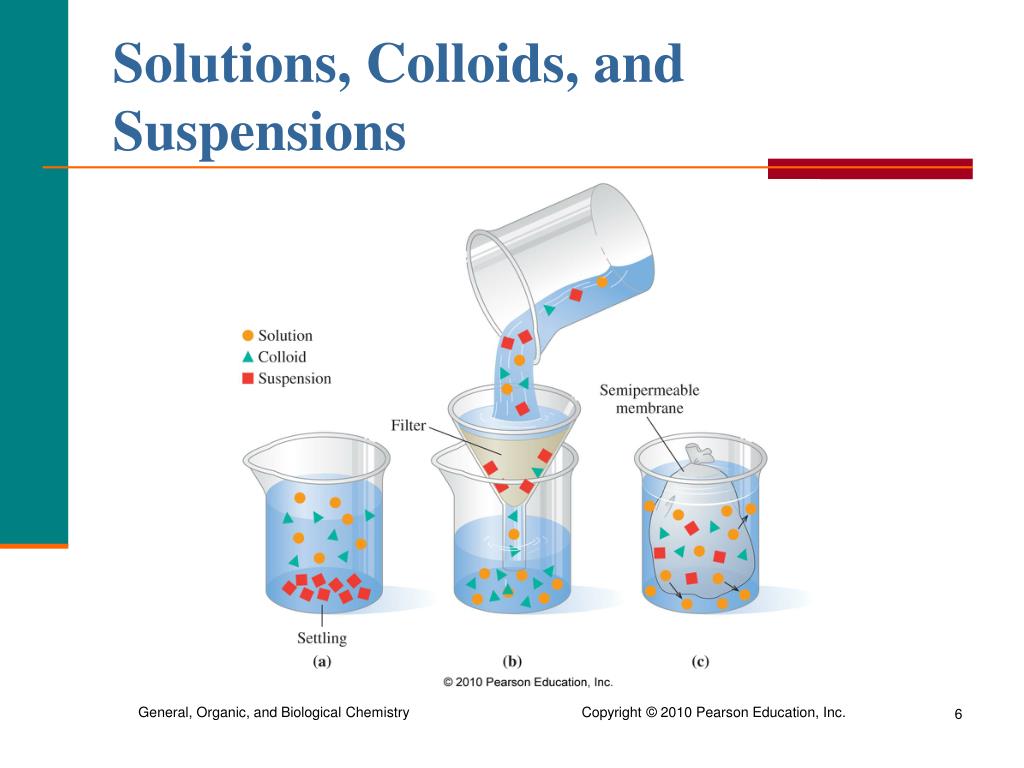
True Solution Suspension And Colloid Difference . The size of particles in a suspension will be greater than 1000 nm. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. Here, the particles are microscopically dispersed and. It is a homogenous mixture where two or more solute dissolves in a solvent. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. Colloids can be distinguished from. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations:
from www.slideserve.com
Here, the particles are microscopically dispersed and. You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. It is a homogenous mixture where two or more solute dissolves in a solvent. Colloids can be distinguished from. The size of particles in a suspension will be greater than 1000 nm. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations:
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID
True Solution Suspension And Colloid Difference Here, the particles are microscopically dispersed and. The size of particles in a suspension will be greater than 1000 nm. Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. Colloids can be distinguished from. It is a homogenous mixture where two or more solute dissolves in a solvent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: A colloidal solution is the mixture of particles of substances that are suspended in the fluid. Here, the particles are microscopically dispersed and.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference True Solution Suspension And Colloid Difference You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. The size of particles in a suspension will be greater than 1000 nm. Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. Here, the particles are microscopically dispersed and. A colloidal solution. True Solution Suspension And Colloid Difference.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz True Solution Suspension And Colloid Difference It is a homogenous mixture where two or more solute dissolves in a solvent. Colloids can be distinguished from. You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: The size of particles in. True Solution Suspension And Colloid Difference.
From giorhfjci.blob.core.windows.net
Colloid Solution Pronunciation at Jodi Bowman blog True Solution Suspension And Colloid Difference You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. It is a homogenous mixture where two or more solute dissolves in a solvent. Colloids can be distinguished from. Colloidal solutions, on the other hand, contain particles that. True Solution Suspension And Colloid Difference.
From www.toppr.com
Which of the following is/are a type of homogenous mixture? True Solution Suspension And Colloid Difference A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The size of particles in a suspension will be greater than 1000 nm. Colloids can be distinguished from. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. Colloidal solutions, on. True Solution Suspension And Colloid Difference.
From www.youtube.com
TRUE SOLUTION COLLOID SUSPENSIONS 10 major differences. YouTube True Solution Suspension And Colloid Difference Colloids can be distinguished from. The size of particles in a suspension will be greater than 1000 nm. Here, the particles are microscopically dispersed and. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. In a mixture, the colloidal solution is one of the significant parts. True Solution Suspension And Colloid Difference.
From brainly.in
[Solved] Distinguish between true solution , colloid and suspension on True Solution Suspension And Colloid Difference A colloidal solution is the mixture of particles of substances that are suspended in the fluid. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The size of particles in a suspension will be greater than 1000 nm. You can tell suspensions from colloids and solutions. True Solution Suspension And Colloid Difference.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs True Solution Suspension And Colloid Difference A colloidal solution is the mixture of particles of substances that are suspended in the fluid. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It is a homogenous mixture where two or more solute dissolves in a solvent. Colloidal solutions, on the other hand, contain. True Solution Suspension And Colloid Difference.
From www.youtube.com
Solution Suspension Colloid YouTube True Solution Suspension And Colloid Difference A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Colloids can be distinguished from. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate.. True Solution Suspension And Colloid Difference.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube True Solution Suspension And Colloid Difference A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It is a homogenous mixture where two or more solute dissolves in a solvent. The size of particles in a suspension will be greater than 1000 nm. In a mixture, the colloidal solution is one of the. True Solution Suspension And Colloid Difference.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's True Solution Suspension And Colloid Difference Colloids can be distinguished from. You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The size of particles in a suspension will be greater than 1000 nm. Here, the particles. True Solution Suspension And Colloid Difference.
From edurev.in
Difference between true solution, suspension and colloidal sol True Solution Suspension And Colloid Difference Colloids can be distinguished from. Here, the particles are microscopically dispersed and. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It is a homogenous mixture where two or more solute dissolves in a solvent. A colloidal solution is the mixture of particles of substances that. True Solution Suspension And Colloid Difference.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download True Solution Suspension And Colloid Difference Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. In a mixture, the colloidal solution is one of the significant parts of the mix with. True Solution Suspension And Colloid Difference.
From gioglbtpv.blob.core.windows.net
Suspension And Colloid And Solution at Jason Vasquez blog True Solution Suspension And Colloid Difference The size of particles in a suspension will be greater than 1000 nm. It is a homogenous mixture where two or more solute dissolves in a solvent. Here, the particles are microscopically dispersed and. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. A colloid is a heterogeneous mixture in which the dispersed. True Solution Suspension And Colloid Difference.
From exooyhgkl.blob.core.windows.net
Suspension Mixture Definition Chemistry at Bill Lance blog True Solution Suspension And Colloid Difference It is a homogenous mixture where two or more solute dissolves in a solvent. The size of particles in a suspension will be greater than 1000 nm. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: You can tell suspensions from colloids and solutions because the components of suspensions. True Solution Suspension And Colloid Difference.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID True Solution Suspension And Colloid Difference Here, the particles are microscopically dispersed and. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. In a mixture, the colloidal solution is one of the significant parts of the mix with. True Solution Suspension And Colloid Difference.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download True Solution Suspension And Colloid Difference Here, the particles are microscopically dispersed and. The size of particles in a suspension will be greater than 1000 nm. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: Colloids can be distinguished from. A colloidal solution is the mixture of particles of substances that are suspended in the. True Solution Suspension And Colloid Difference.
From www.youtube.com
Solution, Suspension and Colloid aumsum kids science education True Solution Suspension And Colloid Difference You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It. True Solution Suspension And Colloid Difference.
From www.studypage.in
True Solution, Colloidal Solution and Suspension Study Page True Solution Suspension And Colloid Difference Colloids can be distinguished from. The size of particles in a suspension will be greater than 1000 nm. It is a homogenous mixture where two or more solute dissolves in a solvent. You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. Here, the particles are microscopically dispersed and. Colloidal solutions, on the other. True Solution Suspension And Colloid Difference.
From hxetzjmqq.blob.core.windows.net
Suspension Colloid Homogeneous at Robert Morris blog True Solution Suspension And Colloid Difference The size of particles in a suspension will be greater than 1000 nm. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Colloids can be distinguished from. Here, the particles. True Solution Suspension And Colloid Difference.
From www.slideshare.net
Colloids True Solution Suspension And Colloid Difference Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. The size of particles in a suspension will be greater than 1000 nm. It is a homogenous mixture where two or more solute dissolves in a solvent. A colloidal solution is the mixture of particles of substances that are. True Solution Suspension And Colloid Difference.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in True Solution Suspension And Colloid Difference A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. Here, the particles are microscopically dispersed and. Colloids can be distinguished from. It is a homogenous. True Solution Suspension And Colloid Difference.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru True Solution Suspension And Colloid Difference It is a homogenous mixture where two or more solute dissolves in a solvent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. In a mixture, the colloidal solution is. True Solution Suspension And Colloid Difference.
From gioglbtpv.blob.core.windows.net
Suspension And Colloid And Solution at Jason Vasquez blog True Solution Suspension And Colloid Difference In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. A. True Solution Suspension And Colloid Difference.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension True Solution Suspension And Colloid Difference You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. The size of particles in a suspension will be greater than 1000 nm. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. Colloids can be distinguished from. It is a homogenous mixture where two or more solute. True Solution Suspension And Colloid Difference.
From giorhfjci.blob.core.windows.net
Colloid Solution Pronunciation at Jodi Bowman blog True Solution Suspension And Colloid Difference A colloidal solution is the mixture of particles of substances that are suspended in the fluid. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Here, the particles are microscopically dispersed and. Colloidal solutions, on the other hand, contain particles that are larger than those in. True Solution Suspension And Colloid Difference.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID True Solution Suspension And Colloid Difference Here, the particles are microscopically dispersed and. The size of particles in a suspension will be greater than 1000 nm. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution. True Solution Suspension And Colloid Difference.
From www.youtube.com
Solutions Suspensions and Colloids Part 1/1 English Class 9 YouTube True Solution Suspension And Colloid Difference You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. Colloids can be distinguished from. In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: A colloidal solution is the mixture of particles of substances that are suspended in the fluid. It is a. True Solution Suspension And Colloid Difference.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube True Solution Suspension And Colloid Difference In a mixture, the colloidal solution is one of the significant parts of the mix with the two adjacent combinations: Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. Here, the particles are microscopically dispersed and. A colloid is a heterogeneous mixture in which the dispersed particles are. True Solution Suspension And Colloid Difference.
From studylib.net
Solutions, Colloids, Suspension True Solution Suspension And Colloid Difference It is a homogenous mixture where two or more solute dissolves in a solvent. Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. The size of particles in a suspension will be greater than 1000 nm. Colloids can be distinguished from. A colloid is a heterogeneous mixture in. True Solution Suspension And Colloid Difference.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of True Solution Suspension And Colloid Difference Colloidal solutions, on the other hand, contain particles that are larger than those in true solutions but smaller than those in. Here, the particles are microscopically dispersed and. Colloids can be distinguished from. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. In a mixture, the colloidal solution is one of the significant. True Solution Suspension And Colloid Difference.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet True Solution Suspension And Colloid Difference The size of particles in a suspension will be greater than 1000 nm. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Here, the particles are microscopically dispersed and. A colloidal solution is the mixture of particles of substances that are suspended in the fluid. In. True Solution Suspension And Colloid Difference.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True True Solution Suspension And Colloid Difference A colloidal solution is the mixture of particles of substances that are suspended in the fluid. Here, the particles are microscopically dispersed and. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The size of particles in a suspension will be greater than 1000 nm. Colloidal. True Solution Suspension And Colloid Difference.