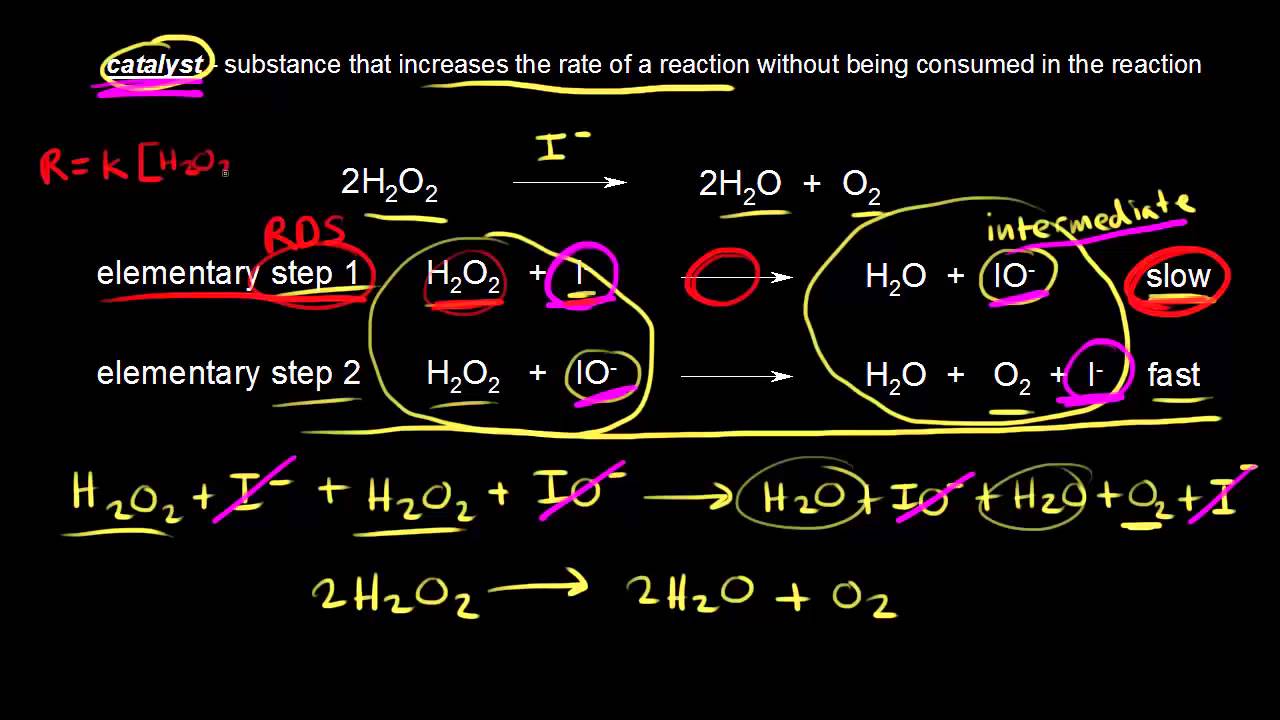
Catalyst Group Chemistry . In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts provide alternative reaction pathways. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. In principal, a catalyst aids transformation of its. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysis is the process of speeding up a reaction using a catalyst. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to. Catalysts are conventionally divided into two categories:
from www.youtube.com
The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. In principal, a catalyst aids transformation of its. Catalysis is the process of speeding up a reaction using a catalyst. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. In general, catalytic action is a chemical reaction between the catalyst and a reactant. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to. Catalysts provide alternative reaction pathways. Catalysts participate in a chemical reaction and increase its rate.
Catalysts AP Chemistry Khan Academy YouTube
Catalyst Group Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts participate in a chemical reaction and increase its rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in the reaction’s net equation and are not consumed during the reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts allow a reaction to. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysis is the process of speeding up a reaction using a catalyst. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. In principal, a catalyst aids transformation of its. Catalysts provide alternative reaction pathways. Catalysts are conventionally divided into two categories: In general, catalytic action is a chemical reaction between the catalyst and a reactant.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Group Chemistry In general, catalytic action is a chemical reaction between the catalyst and a reactant. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts participate in a chemical reaction and increase its rate. Catalysts are conventionally divided into two categories: Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. They do not. Catalyst Group Chemistry.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Group Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysts provide alternative reaction pathways. They. Catalyst Group Chemistry.
From www.nagwa.com
Lesson Catalysts Nagwa Catalyst Group Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts are conventionally divided into two categories: In principal, a catalyst aids transformation of its. Catalysts provide alternative reaction. Catalyst Group Chemistry.
From www.mdpi.com
Catalysts Free FullText The Challenges of Integrating the Catalyst Group Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysts participate in. Catalyst Group Chemistry.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst Group Chemistry In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts provide alternative reaction pathways. Catalysts allow a reaction to. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In general, catalytic action is a chemical reaction between the catalyst and. Catalyst Group Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Group Chemistry Catalysis is the process of speeding up a reaction using a catalyst. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts provide alternative reaction pathways. The word “catalyst” comes from the greek word kataluein, which. Catalyst Group Chemistry.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download Catalyst Group Chemistry Catalysts participate in a chemical reaction and increase its rate. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysts allow a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a. Catalyst Group Chemistry.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Group Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts provide alternative reaction pathways. In principal, a catalyst aids transformation of its. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts are conventionally divided into two categories: In general, catalytic. Catalyst Group Chemistry.
From www.mdpi.com
Catalysts Free FullText Functionalization of Ruthenium Olefin Catalyst Group Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are conventionally divided into two categories: Catalysts provide alternative reaction pathways. Enzymes are naturally occurring catalysts responsible for many essential. Catalyst Group Chemistry.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst Group Chemistry Catalysis is the process of speeding up a reaction using a catalyst. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts provide alternative reaction pathways. In principal, a catalyst aids transformation. Catalyst Group Chemistry.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst Group Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In principal, a catalyst aids transformation of its. Catalysts are conventionally divided into two categories: Catalysts participate in a chemical reaction and increase its rate. This page looks at the the different types of. Catalyst Group Chemistry.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Group Chemistry Catalysis is the process of speeding up a reaction using a catalyst. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts participate in a chemical reaction and increase its rate. Catalysts provide alternative reaction pathways. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts are conventionally divided into two categories:. Catalyst Group Chemistry.
From www.mdpi.com
Catalysts Free FullText Traceless Directing Groups in Sustainable Catalyst Group Chemistry In general, catalytic action is a chemical reaction between the catalyst and a reactant. In principal, a catalyst aids transformation of its. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the. Catalyst Group Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Group Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalyst, in chemistry, any substance that increases the rate of a. Catalyst Group Chemistry.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Group Chemistry In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are conventionally divided into two categories: They do not appear in the reaction’s net equation and are not consumed during the reaction.. Catalyst Group Chemistry.
From www.researchgate.net
2. List of Catalysts Tested and Corresponding Labels Download Table Catalyst Group Chemistry Catalysts are conventionally divided into two categories: Catalysts participate in a chemical reaction and increase its rate. Catalysts provide alternative reaction pathways. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. Catalyst, in chemistry, any substance that increases the rate of a. Catalyst Group Chemistry.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Group Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts participate in a chemical reaction and increase its rate. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and. Catalyst Group Chemistry.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyst Group Chemistry Catalysts provide alternative reaction pathways. In principal, a catalyst aids transformation of its. Catalysis is the process of speeding up a reaction using a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts participate in a chemical reaction and increase its rate. In chemistry and biology, a catalyst is a substance. Catalyst Group Chemistry.
From pubs.acs.org
Frameworks as Catalysts for Organic Synthesis A Critical Catalyst Group Chemistry Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are conventionally divided into two categories: Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. They do not appear in the reaction’s net equation and are not consumed during the. Catalyst Group Chemistry.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst Group Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts allow a reaction to. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind,. Catalyst Group Chemistry.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Group Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. In principal, a catalyst aids transformation of its.. Catalyst Group Chemistry.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Group Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts are conventionally divided into two categories: Catalyst, in chemistry, any substance that increases. Catalyst Group Chemistry.
From www.masterorganicchemistry.com
Olefin Metathesis Master Organic Chemistry Catalyst Group Chemistry Catalysis is the process of speeding up a reaction using a catalyst. Catalysts provide alternative reaction pathways. Catalysts participate in a chemical reaction and increase its rate. In principal, a catalyst aids transformation of its. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts allow a. Catalyst Group Chemistry.
From www.slideserve.com
PPT Basic Chemistry Concepts PowerPoint Presentation, free download Catalyst Group Chemistry Catalysts provide alternative reaction pathways. Catalysis is the process of speeding up a reaction using a catalyst. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry,. Catalyst Group Chemistry.
From www.slideserve.com
PPT Classification of Catalyst Systems PowerPoint Presentation ID Catalyst Group Chemistry In principal, a catalyst aids transformation of its. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts provide alternative reaction pathways. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Enzymes are naturally occurring catalysts responsible for many essential biochemical. Catalyst Group Chemistry.
From chem.unc.edu
Molecular Catalysts Department of Chemistry Catalyst Group Chemistry Catalysts are conventionally divided into two categories: Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts provide alternative reaction pathways. In principal, a catalyst aids transformation of its. In general, catalytic action is a chemical reaction between the catalyst and a reactant.. Catalyst Group Chemistry.
From www.mdpi.com
Catalysts Free FullText RhCatalyzed Environmentally Benign Catalyst Group Chemistry In general, catalytic action is a chemical reaction between the catalyst and a reactant. In principal, a catalyst aids transformation of its. Catalysts participate in a chemical reaction and increase its rate. Catalysts are conventionally divided into two categories: Catalysis is the process of speeding up a reaction using a catalyst. In chemistry and biology, a catalyst is a substance. Catalyst Group Chemistry.
From www.youtube.com
GCSE Chemistry Revision "Catalysts" YouTube Catalyst Group Chemistry Catalysts are conventionally divided into two categories: Catalysts provide alternative reaction pathways. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Enzymes are naturally occurring catalysts responsible for many essential. Catalyst Group Chemistry.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Group Chemistry In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysts participate in a chemical reaction and increase its rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts provide alternative reaction pathways.. Catalyst Group Chemistry.
From www.youtube.com
Types of catalysts AP Chemistry Khan Academy YouTube Catalyst Group Chemistry Catalysis is the process of speeding up a reaction using a catalyst. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. In principal, a catalyst aids transformation of its. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind,. Catalyst Group Chemistry.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Group Chemistry Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to. Catalysis is the process of speeding up a reaction using a catalyst. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. In general, catalytic action is a chemical reaction between the catalyst. Catalyst Group Chemistry.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Group Chemistry Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In principal, a catalyst aids transformation of its. Catalysts provide alternative reaction pathways. Catalysts participate in a chemical reaction and increase its rate. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This page looks at the the different types of catalyst (heterogeneous. Catalyst Group Chemistry.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 Catalyst Group Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts are conventionally divided into two categories: Catalysts allow a reaction to. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts participate in a chemical reaction and increase its rate. The word “catalyst” comes from the. Catalyst Group Chemistry.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Group Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In principal, a catalyst aids transformation of its. In general, catalytic action is a chemical reaction between the catalyst and a reactant. In chemistry and biology, a catalyst is. Catalyst Group Chemistry.
From www.slideserve.com
PPT Surfactants as Catalysts for Organic Reactions in Water Catalyst Group Chemistry Catalysts are conventionally divided into two categories: Catalysts allow a reaction to. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction. Catalyst Group Chemistry.