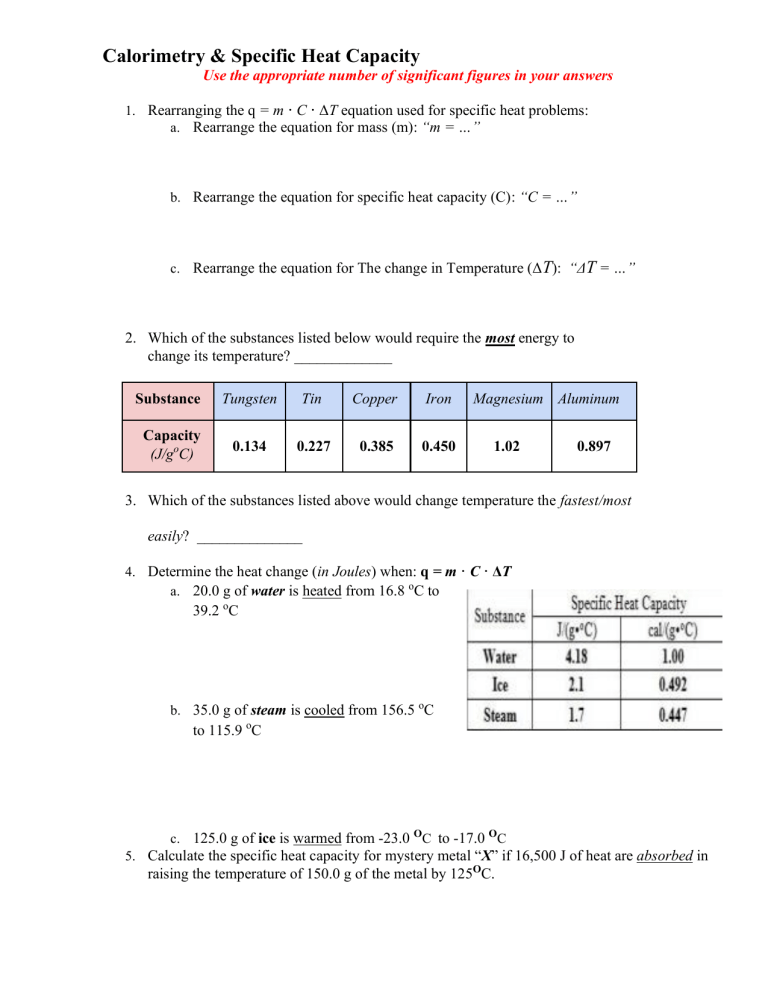
Calorimeter Worksheet . Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. W = p δ v and δ v is zero when the volume is constant. If the combustion of 0.285 moles of this. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. A temperature rise from 25.24°c to 31.67°c was. The specific heat capacity of water is 4.184 j/(g*k). If it takes 683 j of. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Δ e = q + w. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,.
from studylib.net
1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. A temperature rise from 25.24°c to 31.67°c was. The specific heat capacity of water is 4.184 j/(g*k). If it takes 683 j of. If the combustion of 0.285 moles of this. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. Δ e = q + w. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. W = p δ v and δ v is zero when the volume is constant.
Calorimetry Worksheet
Calorimeter Worksheet If it takes 683 j of. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. A temperature rise from 25.24°c to 31.67°c was. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If the combustion of 0.285 moles of this. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. W = p δ v and δ v is zero when the volume is constant. If it takes 683 j of. Δ e = q + w. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. The specific heat capacity of water is 4.184 j/(g*k). So, for a constant volume, δe = q, and therefore, the bomb calorimeter.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimeter Worksheet If it takes 683 j of. The specific heat capacity of water is 4.184 j/(g*k). 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. Δ e = q + w. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. W = p δ v. Calorimeter Worksheet.
From materiallibminauderie.z13.web.core.windows.net
Specific Heat And Calorimetry Worksheet Calorimeter Worksheet Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. A temperature rise from 25.24°c to 31.67°c was. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. If it takes 683 j of. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb. Calorimeter Worksheet.
From ayanahcristien.blogspot.com
20+ Calculating Heat Of Reaction From ConstantPressure Calorimetry Calorimeter Worksheet So, for a constant volume, δe = q, and therefore, the bomb calorimeter. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. If the combustion of 0.285 moles of this. Δ e = q + w. A temperature rise from 25.24°c to 31.67°c was. 1.14 g of octane (c8h18) is combusted. Calorimeter Worksheet.
From studylib.net
Calorimetry Worksheet Calorimeter Worksheet 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If the combustion of 0.285 moles of this. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. If it takes 683 j of. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. A temperature. Calorimeter Worksheet.
From goimages-egg.blogspot.com
Calorimetry Practice Problems Worksheet Answers Goimages Egg Calorimeter Worksheet A temperature rise from 25.24°c to 31.67°c was. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. The specific heat capacity of water is 4.184 j/(g*k). If the combustion of 0.285 moles of this. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Cooh) weighing 1.221 g. Calorimeter Worksheet.
From atanchem.weebly.com
AP Chem Chemistry LHS Calorimeter Worksheet Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. If it takes 683 j of. The specific heat capacity of water is 4.184 j/(g*k). So, for a constant volume, δe = q, and therefore, the bomb calorimeter. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing. Calorimeter Worksheet.
From printablefulltipsy.z13.web.core.windows.net
Heat And Calorimetry Worksheets Calorimeter Worksheet 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. A temperature rise from 25.24°c to 31.67°c was. If it takes 683 j of. W = p δ v and δ v is zero. Calorimeter Worksheet.
From www.studocu.com
Worksheet Chapter 6 calorimetry Key CHEM 1300 General Chemistry I Calorimeter Worksheet A temperature rise from 25.24°c to 31.67°c was. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. W = p δ v and δ v is zero when the volume is constant. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. If the combustion of 0.285 moles. Calorimeter Worksheet.
From www.pinterest.com
Calorimetry Worksheet Answer Key Calorimetry Worksheet Calorie Calorimeter Worksheet If it takes 683 j of. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. The specific heat capacity of water is 4.184 j/(g*k). W = p δ v and δ v is zero when the volume is. Calorimeter Worksheet.
From worksheets.ekocraft-appleleaf.com
Calorimetry Worksheet Chemistry Worksheets For Kindergarten Calorimeter Worksheet 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Δ e = q + w. If it takes 683 j of. The specific heat capacity. Calorimeter Worksheet.
From studylib.net
Calorimetry Worksheet Calorimeter Worksheet The specific heat capacity of water is 4.184 j/(g*k). A temperature rise from 25.24°c to 31.67°c was. Δ e = q + w. If the combustion of 0.285 moles of this. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If. Calorimeter Worksheet.
From www.pinterest.co.uk
Pin on Science Friday Calorimeter Worksheet 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. A temperature rise from 25.24°c to 31.67°c was. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. Δ e =. Calorimeter Worksheet.
From studylib.net
Calorimetry Problems Calorimeter Worksheet A temperature rise from 25.24°c to 31.67°c was. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. The specific heat capacity of water is 4.184 j/(g*k). W = p δ v and δ v is zero when the volume is constant. So, for a constant volume, δe = q, and therefore,. Calorimeter Worksheet.
From studylib.net
Calorimetry practice Calorimeter Worksheet Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. Δ e = q + w. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. A temperature rise from 25.24°c to 31.67°c was. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water.. Calorimeter Worksheet.
From studylib.net
Calorimetry Worksheet Calorimeter Worksheet W = p δ v and δ v is zero when the volume is constant. If the combustion of 0.285 moles of this. The specific heat capacity of water is 4.184 j/(g*k). Δ e = q + w. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. 1) if 0.315 moles of hexane. Calorimeter Worksheet.
From learninglibraryralf.z13.web.core.windows.net
Specific Heat And Calorimetry Worksheet Calorimeter Worksheet 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. W = p δ v and δ v is zero when. Calorimeter Worksheet.
From learningnovotyzy.z21.web.core.windows.net
Heat And Calorimetry Worksheets Calorimeter Worksheet 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. The specific heat capacity of water is 4.184 j/(g*k). Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,.. Calorimeter Worksheet.
From lessondbcrandall.z21.web.core.windows.net
Consonant Blends Worksheet For Kindergarten Calorimeter Worksheet 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If it takes 683 j of. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. The specific heat capacity of water is 4.184 j/(g*k). Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2. Calorimeter Worksheet.
From www.studocu.com
Dry ABC DRY LAB A BOMB CALORIMETRY WORKSHEET A l Work Sheet Do Calorimeter Worksheet Δ e = q + w. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If the combustion of 0.285 moles of this. If it takes 683 j of. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb. Calorimeter Worksheet.
From manualfixfaber.z19.web.core.windows.net
Calorimetry Practice Worksheet Answers Calorimeter Worksheet If the combustion of 0.285 moles of this. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. Δ e = q + w. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. A temperature rise from 25.24°c to 31.67°c was. 1) if 0.315 moles. Calorimeter Worksheet.
From www.studocu.com
Heat Capacity Calorimetry Worksheet answers NAME_________________PER Calorimeter Worksheet The specific heat capacity of water is 4.184 j/(g*k). Δ e = q + w. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. If the combustion of 0.285 moles of this. If it takes. Calorimeter Worksheet.
From chessmuseum.org
50 Calorimetry Worksheet Answer Key Calorimeter Worksheet So, for a constant volume, δe = q, and therefore, the bomb calorimeter. A temperature rise from 25.24°c to 31.67°c was. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. Δ e = q + w. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. If it. Calorimeter Worksheet.
From www.chegg.com
Solved Chapter 6 Calorimetry Worksheet Key Open the Calorimeter Worksheet W = p δ v and δ v is zero when the volume is constant. A temperature rise from 25.24°c to 31.67°c was. Δ e = q + w. If the combustion of 0.285 moles of this. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. 1.14 g of octane (c8h18). Calorimeter Worksheet.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Calorimeter Worksheet A temperature rise from 25.24°c to 31.67°c was. If the combustion of 0.285 moles of this. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Δ e = q + w. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Cooh) weighing 1.221 g was placed in a bomb calorimeter. Calorimeter Worksheet.
From martindxmguide.blogspot.com
32 Chemistry Worksheet Heat And Calorimetry Problems support worksheet Calorimeter Worksheet If it takes 683 j of. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. A temperature rise from 25.24°c to 31.67°c was. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. Δ e. Calorimeter Worksheet.
From study.com
Quiz & Worksheet Calorimetry Calorimeter Worksheet 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. If the combustion of 0.285 moles of this. If it takes 683 j of. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. A temperature. Calorimeter Worksheet.
From studylib.net
Calorimetry Worksheet Calorimeter Worksheet If the combustion of 0.285 moles of this. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. A temperature rise from 25.24°c to 31.67°c was. Δ e = q + w. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. W = p δ v and δ v is. Calorimeter Worksheet.
From chem.libretexts.org
3.4 Calorimetry Chemistry LibreTexts Calorimeter Worksheet Δ e = q + w. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. The specific heat capacity of water is 4.184 j/(g*k). If it takes 683 j of. So, for a constant volume,. Calorimeter Worksheet.
From www.pinterest.com
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library Calorimeter Worksheet Δ e = q + w. If the combustion of 0.285 moles of this. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. The specific heat capacity of water is 4.184 j/(g*k). 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. 1.14 g of octane (c8h18) is combusted in a. Calorimeter Worksheet.
From studylib.net
Calorimetry Worksheets Calorimeter Worksheet If the combustion of 0.285 moles of this. Δ e = q + w. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. The specific heat capacity of water is 4.184 j/(g*k). 1) if 0.315 moles of hexane. Calorimeter Worksheet.
From studylib.net
Calorimetry Worksheet Calorimeter Worksheet 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. A temperature rise from 25.24°c to 31.67°c was. If the combustion of 0.285 moles of this. If it takes 683 j of. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Cooh) weighing 1.221 g was. Calorimeter Worksheet.
From studylib.net
CALORIMETRY WORKSHEET 2 Calorimeter Worksheet 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. A temperature rise from 25.24°c to 31.67°c was. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. If the combustion of 0.285. Calorimeter Worksheet.
From www.onlineworksheet.my.id
Calorimetry Worksheet Answer Key Onlineworksheet.my.id Calorimeter Worksheet If the combustion of 0.285 moles of this. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere.. Calorimeter Worksheet.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimeter Worksheet 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Cooh) weighing 1.221 g was placed in a bomb calorimeter and ignited in a pure o 2 atmosphere. If it takes 683 j of. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. W = p δ v and δ v. Calorimeter Worksheet.
From chem.libretexts.org
6.3 Calorimetry Chemistry LibreTexts Calorimeter Worksheet 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. 1.14 g of octane (c8h18) is combusted in a bomb calorimeter surrounded by 1l of water. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. So, for a constant volume, δe = q, and therefore, the. Calorimeter Worksheet.