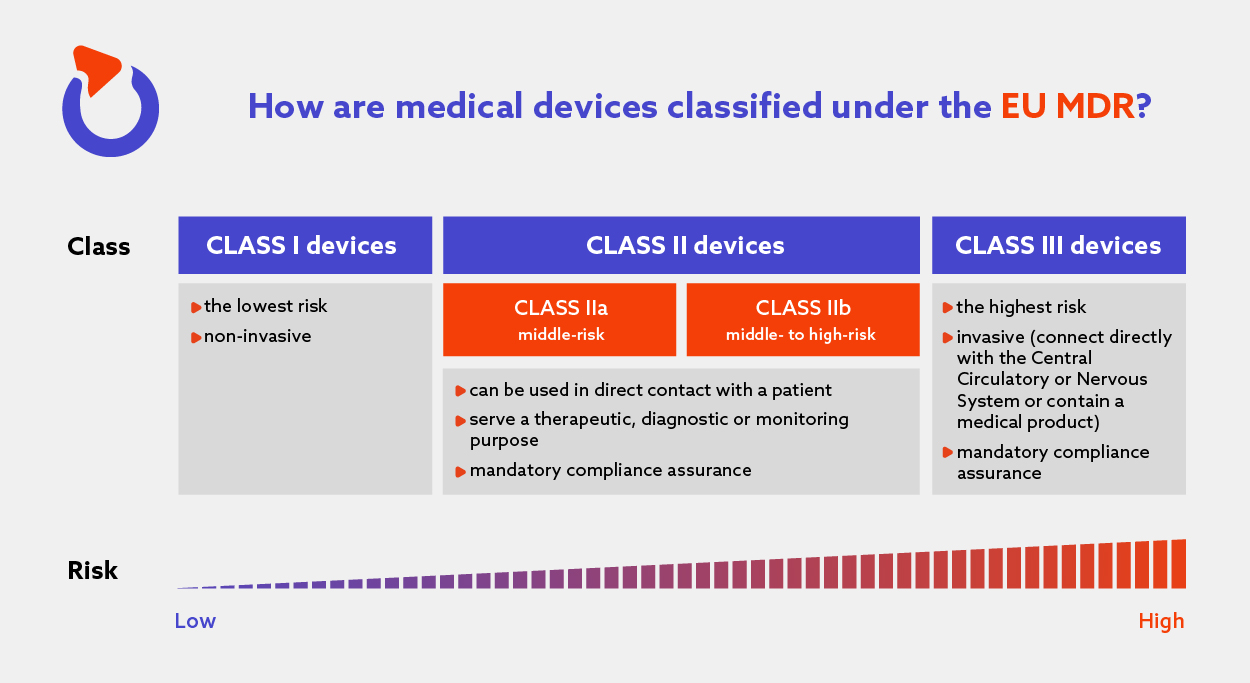
Hearing Aid Medical Device Classification Eu . We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. Scopethis document serves as a guidance document when. Nt manufacturers association annex z 1 published in june 1998. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. Examples of eu mdr class i medical devices include hospital beds,. The mdr medical device classification is based on the device’s potential risk of harm to users. The rules are divided into four sections, and the rules of each section apply to a specific. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of.
from spyro-soft.com
The mdr medical device classification is based on the device’s potential risk of harm to users. Scopethis document serves as a guidance document when. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. Nt manufacturers association annex z 1 published in june 1998. Examples of eu mdr class i medical devices include hospital beds,. The rules are divided into four sections, and the rules of each section apply to a specific. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of.
The Complete Guide to EU Medical Device Regulation Spyrosoft
Hearing Aid Medical Device Classification Eu Nt manufacturers association annex z 1 published in june 1998. Nt manufacturers association annex z 1 published in june 1998. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. The mdr medical device classification is based on the device’s potential risk of harm to users. The rules are divided into four sections, and the rules of each section apply to a specific. Scopethis document serves as a guidance document when. Examples of eu mdr class i medical devices include hospital beds,. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation.
From vem-medical.com
Guide to Medical Device Classification Hearing Aid Medical Device Classification Eu Scopethis document serves as a guidance document when. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. Examples of eu mdr class i medical devices include hospital beds,. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision. Hearing Aid Medical Device Classification Eu.
From easymedicaldevice.com
Complete Guide Medical Device Classification EU MDR (Free PDF) Hearing Aid Medical Device Classification Eu Examples of eu mdr class i medical devices include hospital beds,. Nt manufacturers association annex z 1 published in june 1998. Scopethis document serves as a guidance document when. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. In annex viii of the mdr, you’ll find 22 rules for. Hearing Aid Medical Device Classification Eu.
From www.i3cglobal.com
EU Medical Device Classification Examples and Rules Hearing Aid Medical Device Classification Eu Examples of eu mdr class i medical devices include hospital beds,. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. Scopethis document serves as a guidance document when. Nt manufacturers association annex z 1 published in june 1998. The mdr medical device classification is based on the device’s potential. Hearing Aid Medical Device Classification Eu.
From www.presentationeze.com
EU Medical Device Classification per the EU Directives PresentationEZE Hearing Aid Medical Device Classification Eu We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. Nt manufacturers association annex z 1 published in june 1998. The rules are divided into four sections, and the rules of each section. Hearing Aid Medical Device Classification Eu.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market Hearing Aid Medical Device Classification Eu The rules are divided into four sections, and the rules of each section apply to a specific. Scopethis document serves as a guidance document when. Nt manufacturers association annex z 1 published in june 1998. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. We bring you a second. Hearing Aid Medical Device Classification Eu.
From advanxa.eu
MDR / EUDAMED Advanxa Hearing Aid Medical Device Classification Eu Nt manufacturers association annex z 1 published in june 1998. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. The rules are divided into four sections, and the rules of each section apply to a specific. The mdr medical device classification is based on the device’s potential risk of harm to users. Examples of. Hearing Aid Medical Device Classification Eu.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Hearing Aid Medical Device Classification Eu In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. Examples of eu mdr class i medical devices include hospital beds,. We bring you a second edition of the hearing reimbursement report, looking at what. Hearing Aid Medical Device Classification Eu.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Hearing Aid Medical Device Classification Eu The rules are divided into four sections, and the rules of each section apply to a specific. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. In annex viii. Hearing Aid Medical Device Classification Eu.
From nkyhearing.com
Implantable Hearing Devices Certified Hearing Aid & Audiology Associates Hearing Aid Medical Device Classification Eu Examples of eu mdr class i medical devices include hospital beds,. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. Scopethis document serves as a guidance document when. The classification rules are based on different criteria such as the duration of contact with the patient, the degree. Hearing Aid Medical Device Classification Eu.
From mungfali.com
Classification Of Medical Devices Hearing Aid Medical Device Classification Eu In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. Scopethis document serves as a guidance document when. The mdr medical device classification is based on the device’s potential risk of harm to users. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. Nt. Hearing Aid Medical Device Classification Eu.
From mavink.com
Mdr Classification Chart Hearing Aid Medical Device Classification Eu The rules are divided into four sections, and the rules of each section apply to a specific. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. Examples of eu. Hearing Aid Medical Device Classification Eu.
From easymedicaldevice.com
Complete Guide Medical Device Classification EU MDR (Free PDF) Hearing Aid Medical Device Classification Eu In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. The rules are divided into four sections, and the rules of each section apply to a specific. Examples of eu mdr class i medical devices. Hearing Aid Medical Device Classification Eu.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Hearing Aid Medical Device Classification Eu The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. Scopethis document serves as a guidance document when. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. We bring you a second edition of the hearing reimbursement report, looking at what has changed in. Hearing Aid Medical Device Classification Eu.
From www.youtube.com
Classification Medical Device in EU (Medical Device Regulation MDR 2017 Hearing Aid Medical Device Classification Eu The mdr medical device classification is based on the device’s potential risk of harm to users. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. In annex. Hearing Aid Medical Device Classification Eu.
From www.youtube.com
Classification of Medical Devices EU 2017/745 YouTube Hearing Aid Medical Device Classification Eu This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. The mdr medical device classification is based on the device’s potential risk of harm to users. The rules are divided into four sections, and the rules of each section apply to a specific. Scopethis document serves as a guidance document. Hearing Aid Medical Device Classification Eu.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Hearing Aid Medical Device Classification Eu We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. The mdr medical device classification is based on the device’s potential risk of harm to users. Nt manufacturers association annex z 1 published in june 1998. The rules are divided into four sections, and the rules of each. Hearing Aid Medical Device Classification Eu.
From pepgra.com
Medical Device Classification In The European Union pepgra Hearing Aid Medical Device Classification Eu In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. Examples of eu mdr class i medical devices include hospital beds,. Scopethis document serves as a guidance document when. The rules are divided into four sections, and the rules of each section apply to a specific. Nt manufacturers association annex z 1 published in june. Hearing Aid Medical Device Classification Eu.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Hearing Aid Medical Device Classification Eu In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. Nt manufacturers association annex z 1 published in june 1998. Examples of eu mdr class i medical devices include hospital beds,. The mdr medical device classification is based on the device’s potential risk of harm to users. This guide explains how to classify devices under. Hearing Aid Medical Device Classification Eu.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Hearing Aid Medical Device Classification Eu Examples of eu mdr class i medical devices include hospital beds,. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. The rules are divided into four sections,. Hearing Aid Medical Device Classification Eu.
From lifehealthmax.com
Different Types Of Hearing Aids Life Health Max Hearing Aid Medical Device Classification Eu Scopethis document serves as a guidance document when. Nt manufacturers association annex z 1 published in june 1998. The rules are divided into four sections, and the rules of each section apply to a specific. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. Examples of eu mdr class. Hearing Aid Medical Device Classification Eu.
From www.dreamstime.com
Placement of the Hearing Aid Medical Device Stock Image Image of Hearing Aid Medical Device Classification Eu The rules are divided into four sections, and the rules of each section apply to a specific. Scopethis document serves as a guidance document when. The mdr medical device classification is based on the device’s potential risk of harm to users. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. The classification rules are. Hearing Aid Medical Device Classification Eu.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Hearing Aid Medical Device Classification Eu In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. Nt manufacturers association annex z 1 published in june 1998. The mdr medical device classification is based on the device’s potential risk of harm to users. The rules are divided into four sections, and the rules of each section apply to a specific. This guide. Hearing Aid Medical Device Classification Eu.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Hearing Aid Medical Device Classification Eu Scopethis document serves as a guidance document when. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. The rules are divided into four sections, and the rules of each section apply to a specific. The classification rules are based on different criteria such as the duration of. Hearing Aid Medical Device Classification Eu.
From www.entacochlear.com
Hearing Aid Devices Cochlear Clinic at ENT Associates Hearing Aid Medical Device Classification Eu Examples of eu mdr class i medical devices include hospital beds,. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. Scopethis document serves as a guidance document when. This guide explains how to classify devices under the different medical device classes according to the eu medical devices. Hearing Aid Medical Device Classification Eu.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Hearing Aid Medical Device Classification Eu The mdr medical device classification is based on the device’s potential risk of harm to users. The rules are divided into four sections, and the rules of each section apply to a specific. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. We bring you a second edition of the hearing reimbursement report, looking. Hearing Aid Medical Device Classification Eu.
From emmainternational.com
Classifying Medical Devices under EU MDR Hearing Aid Medical Device Classification Eu Examples of eu mdr class i medical devices include hospital beds,. The mdr medical device classification is based on the device’s potential risk of harm to users. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. This guide explains how to classify devices under the different medical device classes according to the eu medical. Hearing Aid Medical Device Classification Eu.
From bdteletalk.com
Finding The Best Hearing Aid For People With Single Sided Deafness Hearing Aid Medical Device Classification Eu Nt manufacturers association annex z 1 published in june 1998. In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. Examples of eu mdr class i medical devices include hospital beds,. The mdr medical device classification is based on the device’s potential risk of harm to users. We bring you a second edition of the. Hearing Aid Medical Device Classification Eu.
From medicaldevicehq.com
Different classifications rules for medical device software An Hearing Aid Medical Device Classification Eu Nt manufacturers association annex z 1 published in june 1998. The mdr medical device classification is based on the device’s potential risk of harm to users. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. Examples of eu mdr class i medical devices include hospital beds,. In annex viii. Hearing Aid Medical Device Classification Eu.
From meaningkosh.com
Mdr Medical Device Classifications MeaningKosh Hearing Aid Medical Device Classification Eu We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of. Examples of eu mdr class i medical devices include hospital beds,. Scopethis document serves as a guidance document when. Nt manufacturers association annex z 1 published in june 1998. This guide explains how to classify devices under the. Hearing Aid Medical Device Classification Eu.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Hearing Aid Medical Device Classification Eu This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. The mdr medical device classification is based on the device’s potential risk of harm to users. Scopethis document serves as a guidance document when. We bring you a second edition of the hearing reimbursement report, looking at what has changed. Hearing Aid Medical Device Classification Eu.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Hearing Aid Medical Device Classification Eu Scopethis document serves as a guidance document when. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. Nt manufacturers association annex z 1 published in june 1998. We bring you a second edition of the hearing reimbursement report, looking at what has changed in the landscape of provision of.. Hearing Aid Medical Device Classification Eu.
From easymedicaldevice.com
Complete Guide Medical Device Classification EU MDR (Free PDF) Hearing Aid Medical Device Classification Eu The mdr medical device classification is based on the device’s potential risk of harm to users. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. Scopethis document serves as a guidance document when. This guide explains how to classify devices under the different medical device classes according to the. Hearing Aid Medical Device Classification Eu.
From smartdataweek.com
Medical Device Classification (FDA & EU MDR) SimplerQMS (2024) Hearing Aid Medical Device Classification Eu Nt manufacturers association annex z 1 published in june 1998. Scopethis document serves as a guidance document when. Examples of eu mdr class i medical devices include hospital beds,. The rules are divided into four sections, and the rules of each section apply to a specific. The classification rules are based on different criteria such as the duration of contact. Hearing Aid Medical Device Classification Eu.
From operonstrategist.com
Medical Device Classification EU MDR Guide) Operon Strategist Hearing Aid Medical Device Classification Eu In annex viii of the mdr, you’ll find 22 rules for classifying any medical device. This guide explains how to classify devices under the different medical device classes according to the eu medical devices regulation. Examples of eu mdr class i medical devices include hospital beds,. Nt manufacturers association annex z 1 published in june 1998. The classification rules are. Hearing Aid Medical Device Classification Eu.
From omcmedical.com
EU Classification of Medical Devices OMC Medical Hearing Aid Medical Device Classification Eu Examples of eu mdr class i medical devices include hospital beds,. The classification rules are based on different criteria such as the duration of contact with the patient, the degree of. The rules are divided into four sections, and the rules of each section apply to a specific. In annex viii of the mdr, you’ll find 22 rules for classifying. Hearing Aid Medical Device Classification Eu.