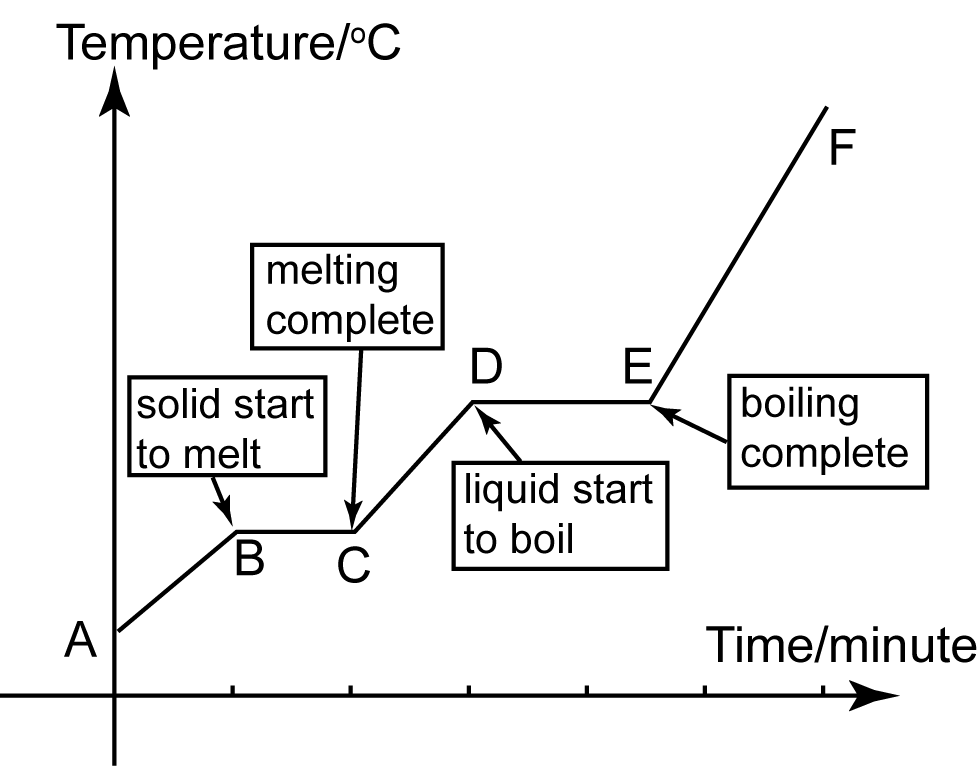
Explain Heating And Cooling Curves Of Water . Heating curves show how the temperature changes as a substance is heated up. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The ice is in a closed container. What are heating and cooling curves? They show how the temperature. Heating and cooling curves are graphs. Heating curves are the graphical correlations between heat added to a substance. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. As heat is steadily added to the ice block, the water. Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point. As heat is steadily added to the ice block, the water. Cooling curves are the opposite. Loss of heat, it is. Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy.
from lessonlibnurselings.z21.web.core.windows.net
They show how the temperature changes as a substance is cooled down. As heat is steadily added to the ice block, the water. They show how the temperature. Heating and cooling curves are graphs. Cooling curves are the opposite. The ice is in a closed container. What are heating and cooling curves? Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point.
Heating And Cooling Curve Explanation
Explain Heating And Cooling Curves Of Water Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. As heat is steadily added to the ice block, the water. Cooling curves are the opposite. Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point. Heating curves show how the temperature changes as a substance is heated up. For heating curves, we start with a solid and add heat energy. Loss of heat, it is. The ice is in a closed container. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. They show how the temperature changes as a substance is cooled down. What are heating and cooling curves? Heating curves are the graphical correlations between heat added to a substance. They show how the temperature. The ice is in a closed container. Heating curves show how the temperature changes as a substance is heated up.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Explain Heating And Cooling Curves Of Water Cooling curves are the opposite. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point. Heating and cooling curves are graphs. Cooling curves are the opposite. Heating curves show. Explain Heating And Cooling Curves Of Water.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Explain Heating And Cooling Curves Of Water As heat is steadily added to the ice block, the water. Cooling curves are the opposite. Cooling curves are the opposite. Heating curves show how the temperature changes as a substance is heated up. Heating curves show how the temperature changes as a substance is heated up. What are heating and cooling curves? When viewed from a cooling perspective, ie.. Explain Heating And Cooling Curves Of Water.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Explain Heating And Cooling Curves Of Water Cooling curves are the opposite. As heat is steadily added to the ice block, the water. When viewed from a cooling perspective, ie. The ice is in a closed container. What are heating and cooling curves? Loss of heat, it is. Cooling curves are the opposite. They show how the temperature. Imagine that you have a block of ice that. Explain Heating And Cooling Curves Of Water.
From study.com
Heating & Cooling Curves Definition, Phases & Examples Lesson Explain Heating And Cooling Curves Of Water Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point. Heating curves show how the temperature changes as a substance is heated up. The ice is in a closed container. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of. Explain Heating And Cooling Curves Of Water.
From www.slideserve.com
PPT Heating and Cooling Curves of Water PowerPoint Presentation, free Explain Heating And Cooling Curves Of Water As heat is steadily added to the ice block, the water. For heating curves, we start with a solid and add heat energy. The ice is in a closed container. They show how the temperature changes as a substance is cooled down. As heat is steadily added to the ice block, the water. Loss of heat, it is. Heating curves. Explain Heating And Cooling Curves Of Water.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Explain Heating And Cooling Curves Of Water Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. The ice is in a closed container. Heating curves are the graphical correlations between heat added to a substance. When. Explain Heating And Cooling Curves Of Water.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Explain Heating And Cooling Curves Of Water Cooling curves are the opposite. As heat is steadily added to the ice block, the water. Cooling curves are the opposite. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. They show how the temperature changes as a substance is cooled down. When viewed from a cooling perspective, ie.. Explain Heating And Cooling Curves Of Water.
From lessonstone.z13.web.core.windows.net
Heating Curve Of Water Explain Heating And Cooling Curves Of Water Heating curves show how the temperature changes as a substance is heated up. As heat is steadily added to the ice block, the water. Cooling curves are the opposite. Cooling curves are the opposite. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. Heating curves are the graphical correlations. Explain Heating And Cooling Curves Of Water.
From amiahgokeestes.blogspot.com
Use the Heating Curve Below to Answer the Following Questions Explain Heating And Cooling Curves Of Water Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point. Heating curves are the graphical correlations between heat added to a substance. They show how the temperature changes as a substance is cooled down. Loss of heat, it is. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature. Explain Heating And Cooling Curves Of Water.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Explain Heating And Cooling Curves Of Water They show how the temperature. Loss of heat, it is. Heating curves are the graphical correlations between heat added to a substance. The ice is in a closed container. Heating curves show how the temperature changes as a substance is heated up. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample. Explain Heating And Cooling Curves Of Water.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Explain Heating And Cooling Curves Of Water Loss of heat, it is. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point. As heat is steadily added to the ice block, the water. The ice is in a. Explain Heating And Cooling Curves Of Water.
From printablelibmolines.z13.web.core.windows.net
Heating Curve Of Water Worksheet Explain Heating And Cooling Curves Of Water As heat is steadily added to the ice block, the water. Loss of heat, it is. Heating curves are the graphical correlations between heat added to a substance. Heating and cooling curves are graphs. As heat is steadily added to the ice block, the water. Imagine that you have a block of ice that is at a temperature of −. Explain Heating And Cooling Curves Of Water.
From www.slideserve.com
PPT Heating/Cooling Curve and Phase Diagrams PowerPoint Presentation Explain Heating And Cooling Curves Of Water What are heating and cooling curves? Heating curves are the graphical correlations between heat added to a substance. As heat is steadily added to the ice block, the water. For heating curves, we start with a solid and add heat energy. As heat is steadily added to the ice block, the water. Imagine that you have a block of ice. Explain Heating And Cooling Curves Of Water.
From slidetodoc.com
CALCULATING ENERGY CHANGES HEATING CURVE OF WATER COOLING Explain Heating And Cooling Curves Of Water Heating and cooling curves are graphs. As heat is steadily added to the ice block, the water. Heating curves show how the temperature changes as a substance is heated up. When viewed from a cooling perspective, ie. What are heating and cooling curves? The ice is in a closed container. Cooling curves are the opposite. Figure \(\pageindex{3}\) shows a heating. Explain Heating And Cooling Curves Of Water.
From printablelibagnames.z13.web.core.windows.net
Heating Curve Of Water Explained Explain Heating And Cooling Curves Of Water Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. When viewed from a cooling perspective, ie. They show how the temperature changes as a substance is cooled down. Heating curves show how the temperature changes as a substance is heated up. They show how the temperature. Heating and cooling. Explain Heating And Cooling Curves Of Water.
From slidetodoc.com
CALCULATING ENERGY CHANGES HEATING CURVE OF WATER COOLING Explain Heating And Cooling Curves Of Water When viewed from a cooling perspective, ie. They show how the temperature. Loss of heat, it is. Cooling curves are the opposite. Heating curves show how the temperature changes as a substance is heated up. As heat is steadily added to the ice block, the water. Heating and cooling curves are graphs. What are heating and cooling curves? The ice. Explain Heating And Cooling Curves Of Water.
From lessonlibnurselings.z21.web.core.windows.net
Heating And Cooling Curve Explanation Explain Heating And Cooling Curves Of Water For heating curves, we start with a solid and add heat energy. Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. The ice is in a closed container.. Explain Heating And Cooling Curves Of Water.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts Explain Heating And Cooling Curves Of Water They show how the temperature. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. When viewed from a cooling perspective, ie. They show how the temperature changes as a substance is cooled down. The ice is in a closed container. As heat is steadily added to the ice block,. Explain Heating And Cooling Curves Of Water.
From lessonlibnurselings.z21.web.core.windows.net
Heating And Cooling Curve Explanation Explain Heating And Cooling Curves Of Water Cooling curves are the opposite. Heating and cooling curves are graphs. Heating curves show how the temperature changes as a substance is heated up. Heating curves are the graphical correlations between heat added to a substance. The ice is in a closed container. When viewed from a cooling perspective, ie. Imagine that you have a block of ice that is. Explain Heating And Cooling Curves Of Water.
From worksheetlistddt.z21.web.core.windows.net
Heating And Cooling Curves Explained Explain Heating And Cooling Curves Of Water Heating and cooling curves are graphs. Loss of heat, it is. The ice is in a closed container. Cooling curves are the opposite. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. Heating curves are the graphical correlations between. Explain Heating And Cooling Curves Of Water.
From www.scribd.com
Heating and Cooling Curves PDF Water Melting Point Explain Heating And Cooling Curves Of Water For heating curves, we start with a solid and add heat energy. As heat is steadily added to the ice block, the water. Heating and cooling curves are graphs. Loss of heat, it is. As heat is steadily added to the ice block, the water. Heating curves provide valuable information about the thermal properties of substances, including their specific heat. Explain Heating And Cooling Curves Of Water.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Explain Heating And Cooling Curves Of Water Heating curves show how the temperature changes as a substance is heated up. As heat is steadily added to the ice block, the water. Imagine that you have a block of ice that is at a temperature of − 30oc, well below its melting point. Heating curves provide valuable information about the thermal properties of substances, including their specific heat. Explain Heating And Cooling Curves Of Water.
From klawvbheb.blob.core.windows.net
Heating And Cooling Curves Diagram at Carl Atkinson blog Explain Heating And Cooling Curves Of Water When viewed from a cooling perspective, ie. Loss of heat, it is. Heating curves show how the temperature changes as a substance is heated up. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. For heating curves, we start with a solid and add heat energy. Heating curves are. Explain Heating And Cooling Curves Of Water.
From www.worldwisetutoring.com
Heating and Cooling Curves Explain Heating And Cooling Curves Of Water Heating curves are the graphical correlations between heat added to a substance. Heating curves show how the temperature changes as a substance is heated up. The ice is in a closed container. Cooling curves are the opposite. Cooling curves are the opposite. As heat is steadily added to the ice block, the water. Heating curves show how the temperature changes. Explain Heating And Cooling Curves Of Water.
From www.youtube.com
Heating and Cooling Curve for Water YouTube Explain Heating And Cooling Curves Of Water Cooling curves are the opposite. As heat is steadily added to the ice block, the water. Loss of heat, it is. They show how the temperature. As heat is steadily added to the ice block, the water. For heating curves, we start with a solid and add heat energy. They show how the temperature changes as a substance is cooled. Explain Heating And Cooling Curves Of Water.
From ch301.cm.utexas.edu
heating curve Explain Heating And Cooling Curves Of Water They show how the temperature. They show how the temperature changes as a substance is cooled down. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. Heating curves are the graphical correlations between heat added to a substance. Imagine that you have a block of ice that is at. Explain Heating And Cooling Curves Of Water.
From classdbjacquelyn.z21.web.core.windows.net
Heat Curve Of Water Explain Heating And Cooling Curves Of Water The ice is in a closed container. Cooling curves are the opposite. Heating curves show how the temperature changes as a substance is heated up. As heat is steadily added to the ice block, the water. The ice is in a closed container. When viewed from a cooling perspective, ie. Cooling curves are the opposite. For heating curves, we start. Explain Heating And Cooling Curves Of Water.
From studylib.net
Heating and Cooling Curves Explain Heating And Cooling Curves Of Water When viewed from a cooling perspective, ie. Heating curves are the graphical correlations between heat added to a substance. Heating curves show how the temperature changes as a substance is heated up. For heating curves, we start with a solid and add heat energy. They show how the temperature. They show how the temperature changes as a substance is cooled. Explain Heating And Cooling Curves Of Water.
From worksheetlistddt.z21.web.core.windows.net
Heating And Cooling Curves Explained Explain Heating And Cooling Curves Of Water As heat is steadily added to the ice block, the water. Heating and cooling curves are graphs. Heating curves show how the temperature changes as a substance is heated up. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature. Explain Heating And Cooling Curves Of Water.
From studyschoolburman.z21.web.core.windows.net
Heating And Cooling Curves Explained Explain Heating And Cooling Curves Of Water For heating curves, we start with a solid and add heat energy. Heating curves show how the temperature changes as a substance is heated up. The ice is in a closed container. What are heating and cooling curves? Heating and cooling curves are graphs. Heating curves show how the temperature changes as a substance is heated up. As heat is. Explain Heating And Cooling Curves Of Water.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Explain Heating And Cooling Curves Of Water They show how the temperature changes as a substance is cooled down. Cooling curves are the opposite. Cooling curves are the opposite. The ice is in a closed container. Loss of heat, it is. Heating curves are the graphical correlations between heat added to a substance. When viewed from a cooling perspective, ie. As heat is steadily added to the. Explain Heating And Cooling Curves Of Water.
From studylib.net
Heating Curve of Water Explain Heating And Cooling Curves Of Water Loss of heat, it is. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heating curves provide valuable information about the thermal properties of substances, including their specific heat capacities, latent heats of fusion. When viewed from a cooling perspective, ie. Cooling curves are the opposite. Heating curves are. Explain Heating And Cooling Curves Of Water.
From lessonlibnurselings.z21.web.core.windows.net
Heating And Cooling Curve Chart Explain Heating And Cooling Curves Of Water Cooling curves are the opposite. The ice is in a closed container. Cooling curves are the opposite. As heat is steadily added to the ice block, the water. Heating curves show how the temperature changes as a substance is heated up. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of. Explain Heating And Cooling Curves Of Water.
From www.showme.com
Heating and Cooling Curves Explained Science, Heating Curve, Cooling Explain Heating And Cooling Curves Of Water Cooling curves are the opposite. They show how the temperature. Heating curves show how the temperature changes as a substance is heated up. What are heating and cooling curves? As heat is steadily added to the ice block, the water. For heating curves, we start with a solid and add heat energy. Heating curves are the graphical correlations between heat. Explain Heating And Cooling Curves Of Water.
From www.slideserve.com
PPT Heating and Cooling Curves of Water PowerPoint Presentation ID Explain Heating And Cooling Curves Of Water The ice is in a closed container. Loss of heat, it is. Heating curves are the graphical correlations between heat added to a substance. Heating curves show how the temperature changes as a substance is heated up. As heat is steadily added to the ice block, the water. The ice is in a closed container. Heating curves show how the. Explain Heating And Cooling Curves Of Water.