Medical Device Environmental Testing Standards . this document, which includes the essential principles of safety and performance, identifies significant standards and guides. More than 80 percent of hospitals. standards for medical devices in japan. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. industrial sterilization and contamination control programs are critical to medical device manufacturing. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. iso 14971 specifies the process for risk management of medical devices, software as a. [2024/08/15] <md>information about standards is.
from studylib.net
standards for medical devices in japan. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. industrial sterilization and contamination control programs are critical to medical device manufacturing. iso 14971 specifies the process for risk management of medical devices, software as a. [2024/08/15] <md>information about standards is. More than 80 percent of hospitals.
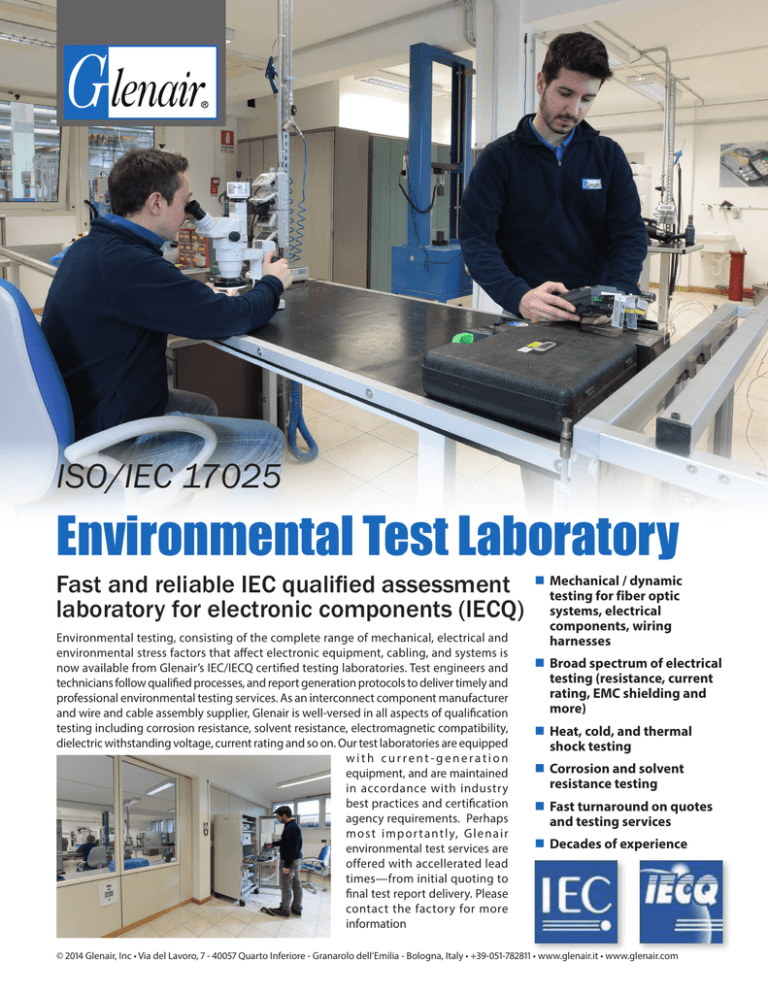
Environmental Test Laboratory
Medical Device Environmental Testing Standards industrial sterilization and contamination control programs are critical to medical device manufacturing. iso 14971 specifies the process for risk management of medical devices, software as a. standards for medical devices in japan. industrial sterilization and contamination control programs are critical to medical device manufacturing. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. More than 80 percent of hospitals. [2024/08/15] <md>information about standards is.
From www.nqa.com
What Standards Apply to Medical Devices Manufacturing? NQA Medical Device Environmental Testing Standards standards for medical devices in japan. [2024/08/15] <md>information about standards is. iso 14971 specifies the process for risk management of medical devices, software as a. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. industrial sterilization and contamination control programs. Medical Device Environmental Testing Standards.
From whia.com.au
Understanding the top benefits of environmental testing services Medical Device Environmental Testing Standards standards for medical devices in japan. More than 80 percent of hospitals. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. industrial sterilization and contamination control programs are critical to medical device manufacturing. iso 14971 specifies the process for risk management of medical devices, software as a. patients. Medical Device Environmental Testing Standards.
From www.greenlight.guru
Standards Makes the Medical Device World go Around Medical Device Environmental Testing Standards [2024/08/15] <md>information about standards is. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. industrial sterilization and contamination control programs are critical to medical device. Medical Device Environmental Testing Standards.
From www.smithersapex.com
Medical Device Testing Video Smithers Medical Device Environmental Testing Standards [2024/08/15] <md>information about standards is. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. standards for medical devices in japan. industrial sterilization and contamination control programs are critical to medical device manufacturing. More than 80 percent of hospitals. iso 14971 specifies the process for risk management of medical devices,. Medical Device Environmental Testing Standards.
From www.cirs-group.com
Testing Testing Medical Devices Medical Device Environmental Testing Standards this document, which includes the essential principles of safety and performance, identifies significant standards and guides. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. standards for medical devices in japan. [2024/08/15] <md>information about standards is. industrial sterilization and contamination control programs are critical. Medical Device Environmental Testing Standards.
From www.qualityfirstint.com
QFIS 0032022 Medical device environmental (waste) management system Medical Device Environmental Testing Standards this document, which includes the essential principles of safety and performance, identifies significant standards and guides. [2024/08/15] <md>information about standards is. iso 14971 specifies the process for risk management of medical devices, software as a. More than 80 percent of hospitals. standards for medical devices in japan. widely used across the industry, iso 14155 has been. Medical Device Environmental Testing Standards.
From www.tentamus.com
Medical Device Testing Tentamus Group Medical Device Environmental Testing Standards widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. industrial sterilization and contamination control programs are critical to medical device manufacturing. More than 80 percent of hospitals. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. iso. Medical Device Environmental Testing Standards.
From www.researchgate.net
List of medical device standards to be harmonized Download Scientific Medical Device Environmental Testing Standards this document, which includes the essential principles of safety and performance, identifies significant standards and guides. standards for medical devices in japan. More than 80 percent of hospitals. [2024/08/15] <md>information about standards is. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. patients and. Medical Device Environmental Testing Standards.
From www.qatestinglaboratories.com
What are the benefits of an Environmental Testing Laboratory? qatesting Medical Device Environmental Testing Standards widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. iso 14971 specifies the process for risk management of medical devices, software as a. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must. Medical Device Environmental Testing Standards.
From www.greenlight.guru
ISO Standards for Medical Devices Ultimate List & Overview Medical Device Environmental Testing Standards patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. More than 80 percent of hospitals. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. standards for medical devices in. Medical Device Environmental Testing Standards.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Medical Device Environmental Testing Standards More than 80 percent of hospitals. industrial sterilization and contamination control programs are critical to medical device manufacturing. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. iso 14971 specifies the process for risk management of medical devices, software as a.. Medical Device Environmental Testing Standards.
From technologydesk.site
Medical Device Standards Purpose And Popular Examples TechnologyDesk Medical Device Environmental Testing Standards iso 14971 specifies the process for risk management of medical devices, software as a. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. [2024/08/15] <md>information about standards is. . Medical Device Environmental Testing Standards.
From studylib.net
Environmental Test Laboratory Medical Device Environmental Testing Standards standards for medical devices in japan. industrial sterilization and contamination control programs are critical to medical device manufacturing. iso 14971 specifies the process for risk management of medical devices, software as a. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. More than 80. Medical Device Environmental Testing Standards.
From www.ppsthane.com
Environmental Testing Laboratory Perfect Pollucon Services Medical Device Environmental Testing Standards [2024/08/15] <md>information about standards is. industrial sterilization and contamination control programs are critical to medical device manufacturing. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. More than 80 percent of hospitals. standards for medical devices in japan. iso 14971. Medical Device Environmental Testing Standards.
From www.en-standard.eu
BS EN 6006812014 Environmental testing General and guidance Medical Device Environmental Testing Standards iso 14971 specifies the process for risk management of medical devices, software as a. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. More than 80 percent of hospitals. patients and users rely on the safety of your medical devices, so conformity with the applicable. Medical Device Environmental Testing Standards.
From senzagen.com
Overview Biological Evaluation of Medical Devices Senzagen Medical Device Environmental Testing Standards iso 14971 specifies the process for risk management of medical devices, software as a. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. standards for medical devices in japan. widely used across the industry, iso 14155 has been revised to. Medical Device Environmental Testing Standards.
From www.ul.com
Medical Laboratory Equipment Testing to the IEC 61010 Standard UL Medical Device Environmental Testing Standards patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. More than 80 percent of hospitals. industrial sterilization and contamination control programs are critical to medical device manufacturing. iso 14971 specifies the process for risk management of medical devices, software as a.. Medical Device Environmental Testing Standards.
From www.testlabs.my
Environmental Testing That Every R&D Engineer Must Know TESTLABS.MY Medical Device Environmental Testing Standards widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. [2024/08/15] <md>information about standards is. this document, which includes the essential. Medical Device Environmental Testing Standards.
From dynateklabs.com
Medical Device Testing Standards Dynatek Labs Medical Device Environmental Testing Standards standards for medical devices in japan. industrial sterilization and contamination control programs are critical to medical device manufacturing. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. [2024/08/15] <md>information about standards is. this document, which includes the essential principles of. Medical Device Environmental Testing Standards.
From www.globaltestinglabs.com
Environmental Testing Services Global Testing Laboratories Medical Device Environmental Testing Standards industrial sterilization and contamination control programs are critical to medical device manufacturing. More than 80 percent of hospitals. iso 14971 specifies the process for risk management of medical devices, software as a. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. widely used across the industry, iso 14155 has. Medical Device Environmental Testing Standards.
From www.testups.com
Medical Device Testing Testups Testing, Certification Medical Device Environmental Testing Standards standards for medical devices in japan. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. industrial sterilization and contamination control programs are critical to medical device manufacturing. iso 14971 specifies the process for risk management of medical devices, software as a. More than 80. Medical Device Environmental Testing Standards.
From www.productsafetylabs.com
Medical Device Testing Medical Device Environmental Testing Standards patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. [2024/08/15] <md>information about standards is. standards for medical devices in japan.. Medical Device Environmental Testing Standards.
From www.needmagazine.com
Exploring the Different Types of Environmental Test ChambersNeed Magazine Medical Device Environmental Testing Standards [2024/08/15] <md>information about standards is. iso 14971 specifies the process for risk management of medical devices, software as a. industrial sterilization and contamination control programs are critical to medical device manufacturing. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. patients and users rely on the safety of your. Medical Device Environmental Testing Standards.
From whiteoutpress.com
The Role of Standards and Regulations in Environmental Testing (2024 Medical Device Environmental Testing Standards standards for medical devices in japan. iso 14971 specifies the process for risk management of medical devices, software as a. More than 80 percent of hospitals. [2024/08/15] <md>information about standards is. industrial sterilization and contamination control programs are critical to medical device manufacturing. this document, which includes the essential principles of safety and performance, identifies significant. Medical Device Environmental Testing Standards.
From technonguide.com
Medical Device Testing Overview and Types Technonguide Medical Device Environmental Testing Standards industrial sterilization and contamination control programs are critical to medical device manufacturing. More than 80 percent of hospitals. standards for medical devices in japan. [2024/08/15] <md>information about standards is. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. patients and users rely on the. Medical Device Environmental Testing Standards.
From www.atmarstech.com
Medical Equipment Reliability and Environmental Testing Medical Device Environmental Testing Standards patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. More than 80 percent of hospitals. iso 14971 specifies the process for risk management of medical. Medical Device Environmental Testing Standards.
From info.dicksondata.com
Environmental Monitoring How to Maintain Compliance Dickson Medical Device Environmental Testing Standards industrial sterilization and contamination control programs are critical to medical device manufacturing. iso 14971 specifies the process for risk management of medical devices, software as a. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. standards for medical devices in japan. patients and. Medical Device Environmental Testing Standards.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Medical Device Environmental Testing Standards this document, which includes the essential principles of safety and performance, identifies significant standards and guides. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. [2024/08/15] <md>information about standards is. More than 80 percent of hospitals. iso 14971 specifies the process. Medical Device Environmental Testing Standards.
From www.mysgi.in
Environmental Testing Services Safeguard International Medical Device Environmental Testing Standards standards for medical devices in japan. More than 80 percent of hospitals. iso 14971 specifies the process for risk management of medical devices, software as a. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. patients and users rely on the safety of your. Medical Device Environmental Testing Standards.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Environmental Testing Standards patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. More than 80 percent of hospitals. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. iso 14971 specifies the process. Medical Device Environmental Testing Standards.
From www.gmdesigndevelopment.com
MEDICAL DEVICE STANDARDS Gm Design Development UK Medical Device Environmental Testing Standards iso 14971 specifies the process for risk management of medical devices, software as a. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. patients and users rely on. Medical Device Environmental Testing Standards.
From www.pinterest.at
ISO 13485 Basics and How to Get Started (QMS for Medical Devices Medical Device Environmental Testing Standards widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. iso 14971 specifies the process for risk management of medical devices,. Medical Device Environmental Testing Standards.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Medical Device Environmental Testing Standards patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. widely used across the industry, iso 14155 has been revised to align with recent regulatory changes and amendments to other relevant. iso 14971 specifies the process for risk management of medical devices,. Medical Device Environmental Testing Standards.
From www.digitalsys.com
MILSTD810 Environmental Testing Standards for Rugged Electronics Medical Device Environmental Testing Standards standards for medical devices in japan. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. More than 80 percent of hospitals. widely used across. Medical Device Environmental Testing Standards.
From www.greenlight.guru
Ultimate Guide to ISO 13485 Quality Management System (QMS) for Medical Medical Device Environmental Testing Standards More than 80 percent of hospitals. this document, which includes the essential principles of safety and performance, identifies significant standards and guides. patients and users rely on the safety of your medical devices, so conformity with the applicable requirements of the target market must be clearly ensured. standards for medical devices in japan. widely used across. Medical Device Environmental Testing Standards.