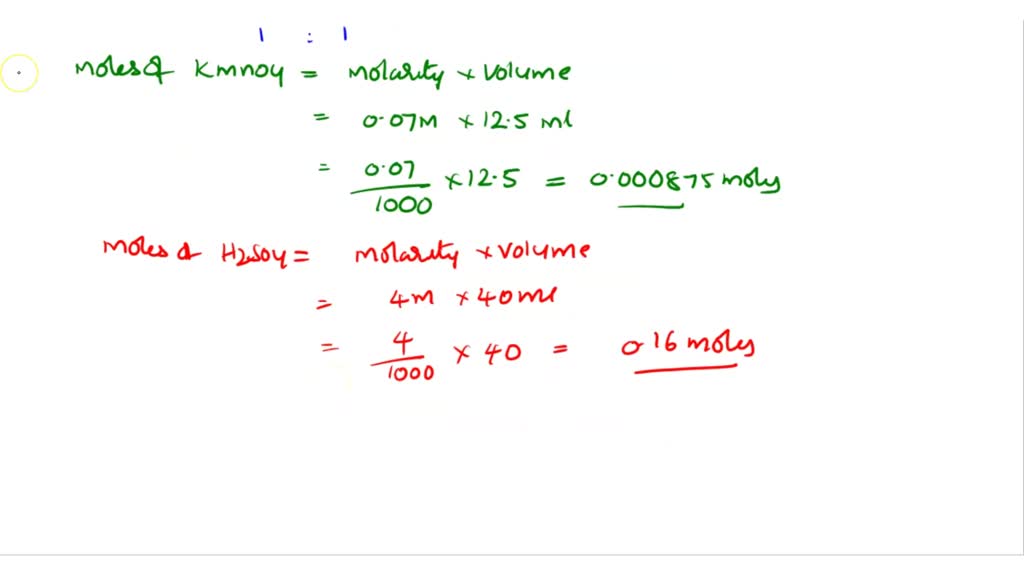
H2O2 Titration With Kmno4 Equation . Calculate percent composition (by mass) of the sample. __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. Use the equation below (balance it):. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. There are two possible ways to balance the following reaction equation: Kmno4 titration is a redox titration method that involves the use. What is the content of hydrogen peroxide? Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. How does kmno4 titration work in determining h2o2 concentration?
from www.numerade.com
There are two possible ways to balance the following reaction equation: Kmno4 titration is a redox titration method that involves the use. Use the equation below (balance it):. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. What is the content of hydrogen peroxide? How does kmno4 titration work in determining h2o2 concentration? Calculate percent composition (by mass) of the sample. The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide.
SOLVED Consider the following chemical equations describing the
H2O2 Titration With Kmno4 Equation Use the equation below (balance it):. Kmno4 titration is a redox titration method that involves the use. __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. There are two possible ways to balance the following reaction equation: This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Calculate percent composition (by mass) of the sample. Use the equation below (balance it):. How does kmno4 titration work in determining h2o2 concentration? One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. What is the content of hydrogen peroxide?
From www.numerade.com
In a titration experiment, H2O2(aq) reacts with aqueous MnO4^(aq) as H2O2 Titration With Kmno4 Equation How does kmno4 titration work in determining h2o2 concentration? There are two possible ways to balance the following reaction equation: This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED 100 ml of given KMnO4 solution titrates 10 ml of 0.1 m oxalic H2O2 Titration With Kmno4 Equation Calculate percent composition (by mass) of the sample. There are two possible ways to balance the following reaction equation: This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Kmno4 titration is a redox titration method that involves the use. __ h 2 o 2 (aq) + 2 kmno 4 (aq). H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium H2O2 Titration With Kmno4 Equation __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. How does kmno4 titration work in determining h2o2 concentration? The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. Hydrogen peroxide. H2O2 Titration With Kmno4 Equation.
From www.flinnsci.com
Analysis of Hydrogen Peroxide A Redox Titration—ChemTopic™ Lab H2O2 Titration With Kmno4 Equation The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. What is the content of hydrogen peroxide? Calculate percent composition (by mass) of the sample. There are two possible ways to balance the following reaction equation: Use the equation below (balance it):. One method. H2O2 Titration With Kmno4 Equation.
From www.chegg.com
Solved Table 2 Titration of H2O2(aq) with KMnO4(aq) (Volume H2O2 Titration With Kmno4 Equation Kmno4 titration is a redox titration method that involves the use. Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. Calculate percent composition (by mass) of the sample. How does kmno4 titration work in determining h2o2 concentration? __ h 2 o 2 (aq) + 2 kmno 4 (aq). H2O2 Titration With Kmno4 Equation.
From www.chegg.com
Solved Data Table 1 Titration of 1.00 mL H2O2 with KMnO4 H2O2 Titration With Kmno4 Equation This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Calculate percent composition (by mass) of the sample. Use the equation below (balance it):. Kmno4 titration is a redox titration method that involves the use. The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution,. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED KMnO4 and Na2C2O4 solutions were used in the reactions that H2O2 Titration With Kmno4 Equation Kmno4 titration is a redox titration method that involves the use. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. Calculate percent composition (by mass) of the sample. Hydrogen peroxide is usually treated as a strong oxidizer, but in the. H2O2 Titration With Kmno4 Equation.
From byjus.com
10.20 ml H202 sol. React completely with 20ml of KMnO4 sol. Under H2O2 Titration With Kmno4 Equation There are two possible ways to balance the following reaction equation: Calculate percent composition (by mass) of the sample. Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. What is the content of hydrogen peroxide? This method is designed for the determination of hydrogen peroxide in aqueous solutions. H2O2 Titration With Kmno4 Equation.
From www.youtube.com
Calculation of KMnO4 and Oxalic acid titration YouTube H2O2 Titration With Kmno4 Equation The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. Kmno4 titration is a redox titration method that involves the use. Use the equation below (balance it):. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED Consider the following chemical equations describing the H2O2 Titration With Kmno4 Equation This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. There are two possible ways to balance the following reaction equation: Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. Use the equation below (balance it):. __ h 2. H2O2 Titration With Kmno4 Equation.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 H2O2 Titration With Kmno4 Equation One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. There are two possible ways. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
VIDEO solution Part C Titration of acidic H2O2 with the KMnO4 H2O2 Titration With Kmno4 Equation __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. Calculate percent composition (by mass) of the sample. What is the content of hydrogen peroxide? One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of. H2O2 Titration With Kmno4 Equation.
From www.toppr.com
15. H2O2 + KMnO4 = MnO2 + KOH+ O2 + H2O H2O2 Titration With Kmno4 Equation Kmno4 titration is a redox titration method that involves the use. There are two possible ways to balance the following reaction equation: Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70%. H2O2 Titration With Kmno4 Equation.
From www.youtube.com
How to Write the Net Ionic Equation for H2O2 + KMnO4 + H2SO4 YouTube H2O2 Titration With Kmno4 Equation Use the equation below (balance it):. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. What is the content of hydrogen. H2O2 Titration With Kmno4 Equation.
From www.chegg.com
Solved Data Table 1 Titration of 1.00 mL H2O2 with KMnO4 H2O2 Titration With Kmno4 Equation Use the equation below (balance it):. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Calculate percent composition (by mass) of. H2O2 Titration With Kmno4 Equation.
From www.slideserve.com
PPT Analysis of Hydrogen Peroxide (redox titration) PowerPoint H2O2 Titration With Kmno4 Equation Calculate percent composition (by mass) of the sample. What is the content of hydrogen peroxide? __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. Use the equation below (balance it):. There are two possible ways to balance the following reaction equation: Hydrogen peroxide is usually treated. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
VIDEO solution Part C Titration of acidic H2O2 with the KMnO4 H2O2 Titration With Kmno4 Equation Use the equation below (balance it):. How does kmno4 titration work in determining h2o2 concentration? Calculate percent composition (by mass) of the sample. __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. The kmno4 solution is purple in color, as it is added to the hydrogen. H2O2 Titration With Kmno4 Equation.
From www.youtube.com
KMnO4 + H2O2 YouTube H2O2 Titration With Kmno4 Equation One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. Calculate percent composition (by mass). H2O2 Titration With Kmno4 Equation.
From mavink.com
H Kmno4 H2O2 Titration With Kmno4 Equation What is the content of hydrogen peroxide? There are two possible ways to balance the following reaction equation: This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. How does kmno4 titration work in determining h2o2 concentration? __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h. H2O2 Titration With Kmno4 Equation.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download H2O2 Titration With Kmno4 Equation Use the equation below (balance it):. Kmno4 titration is a redox titration method that involves the use. There are two possible ways to balance the following reaction equation: How does kmno4 titration work in determining h2o2 concentration? Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. This method. H2O2 Titration With Kmno4 Equation.
From rdsic.edu.vn
Phản ứng KMnO4 H2O2 H2SO4 Công Dụng và Quy Trình Thực Hiện H2O2 Titration With Kmno4 Equation Calculate percent composition (by mass) of the sample. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide is usually. H2O2 Titration With Kmno4 Equation.
From www.youtube.com
General Chemistry Standardization of KMnO4 By Redox Titration YouTube H2O2 Titration With Kmno4 Equation How does kmno4 titration work in determining h2o2 concentration? Use the equation below (balance it):. Calculate percent composition (by mass) of the sample. __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. There are two possible ways to balance the following reaction equation: Hydrogen peroxide is. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
A particle view of a sample of H2O2(aq) is shown above. The H2O2(aq) is H2O2 Titration With Kmno4 Equation Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. What is the content of hydrogen peroxide? Kmno4 titration is a redox titration method that involves the use. __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g). H2O2 Titration With Kmno4 Equation.
From www.slideshare.net
14 titration of h2 o2 H2O2 Titration With Kmno4 Equation Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. There are two possible ways to balance the following reaction equation: __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. Use the equation below (balance. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium H2O2 Titration With Kmno4 Equation The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. Kmno4 titration is a redox titration method that involves the use. How does kmno4 titration work in determining h2o2 concentration? Calculate percent composition (by mass) of the sample. __ h 2 o 2 (aq). H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED KMnO4 (Potassium permanganate) = 4.19 (m/m) H2O2 (Hydrogen H2O2 Titration With Kmno4 Equation __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. What is the content of hydrogen peroxide? Use the equation below (balance it):. The kmno4 solution is purple. H2O2 Titration With Kmno4 Equation.
From www.youtube.com
Titration of KMnO4 by H2O2 with proportional pipettor YouTube H2O2 Titration With Kmno4 Equation __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. How does kmno4 titration work in determining h2o2 concentration? The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+, and. Hydrogen peroxide. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED The amount of hydrogen peroxide (H2O2) in a sample of hair H2O2 Titration With Kmno4 Equation Kmno4 titration is a redox titration method that involves the use. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED 3.[/6 Points] DETAILS MY NOTES ASK YOUR TEACHER Consider a H2O2 Titration With Kmno4 Equation Calculate percent composition (by mass) of the sample. Use the equation below (balance it):. __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. Kmno4 titration is a redox titration method that involves the use. What is the content of hydrogen peroxide? The kmno4 solution is purple. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED (b) Complete the following table for this titration. Data Table H2O2 Titration With Kmno4 Equation Use the equation below (balance it):. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. The kmno4 solution is purple in color, as it is added to the hydrogen peroxide solution, it reacts with the h2o2 to produce colorless mn2+,. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED A certain amount of hydrogen peroxide was dissolved in 100. mL H2O2 Titration With Kmno4 Equation Kmno4 titration is a redox titration method that involves the use. Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. Calculate percent composition (by mass) of the sample. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a. H2O2 Titration With Kmno4 Equation.
From byjus.com
0.2 g sample of H2O2 required 10 ml of 1N KMnO4 for complete reaction H2O2 Titration With Kmno4 Equation There are two possible ways to balance the following reaction equation: Use the equation below (balance it):. What is the content of hydrogen peroxide? Kmno4 titration is a redox titration method that involves the use. Hydrogen peroxide is usually treated as a strong oxidizer, but in the presence of even stronger oxidizer it can become a. Calculate percent composition (by. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium H2O2 Titration With Kmno4 Equation There are two possible ways to balance the following reaction equation: How does kmno4 titration work in determining h2o2 concentration? Calculate percent composition (by mass) of the sample. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. The kmno4 solution. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED For a titration of H2O2 with KMnO4, considering that you have a H2O2 Titration With Kmno4 Equation __ h 2 o 2 (aq) + 2 kmno 4 (aq) + 3 h 2 so 4 (aq) __o 2 (g) + 2. Use the equation below (balance it):. Calculate percent composition (by mass) of the sample. Kmno4 titration is a redox titration method that involves the use. There are two possible ways to balance the following reaction equation: Hydrogen. H2O2 Titration With Kmno4 Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of KMnO4 H2O2 Titration With Kmno4 Equation There are two possible ways to balance the following reaction equation: One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. What is the content of hydrogen peroxide? How does kmno4 titration work in determining h2o2 concentration? This method is designed. H2O2 Titration With Kmno4 Equation.