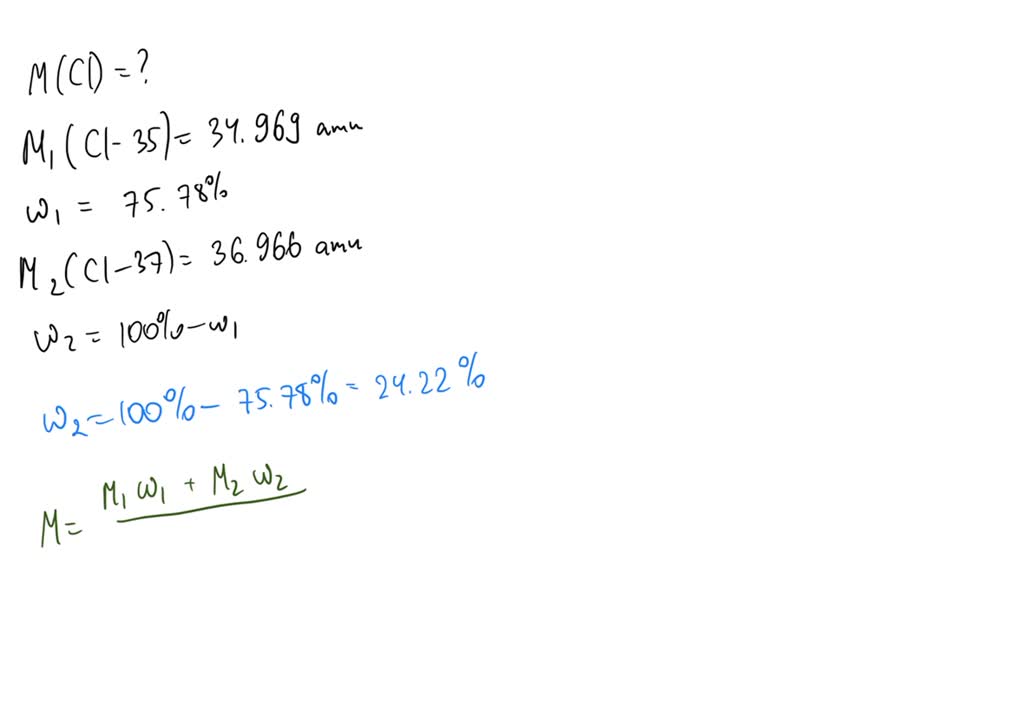Chlorine Has Two Isotopes . The relative abundance of an isotope is 0.755 and. These are its only two natural isotopes occurring in quantity, with 35 cl making up 76% of natural chlorine. chlorine has two stable isotopes: The chlorine has multiple isotopes and is hit with a. Chlorine is taken as typical of elements with more than one atom per molecule. Chlorine has two stable isotopes: the mass spectrum of chlorine. find the average atomic mass of chlorine. The relative abundance of an isotope is 0.755 and. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other. naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of. 75.77% of chlorine is cl35, and 24.23% is cl37. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Unlike protons, the number of neutrons is not absolutely fixed for most elements. chlorine has two stable isotopes, 35 cl and 37 cl.
from www.numerade.com
There are two principal stable. 75.77% of chlorine is cl35, and 24.23% is cl37. There are only two stable isotopes: The relative abundances of these two isotopes. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other. The chlorine has multiple isotopes and is hit with a. We'll look at its mass spectrum to show. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of. This is because chlorine contains two.
SOLVED Chlorine has two naturally occuring isotopes chlorine35 and
Chlorine Has Two Isotopes The relative abundance of an isotope is 0.755 and. all atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. chlorine has two stable isotopes: Chlorine is taken as typical of elements with more than one atom per molecule. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks. We'll look at its mass spectrum to show. (a) when a sample of elemental chlorine is injected into. The relative abundance of an isotope is 0.755 and. These are its only two natural isotopes occurring in quantity, with 35 cl making up 76% of natural chlorine. Unlike protons, the number of neutrons is not absolutely fixed for most elements. naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are only two stable isotopes: Chlorine has two isotopes of atomic mass units 34.97 and 36.97. The relative abundance of an isotope is 0.755 and. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl.
From www.slideserve.com
PPT 1. Atomic Structure PowerPoint Presentation, free download ID Chlorine Has Two Isotopes Chlorine has two isotopes of atomic mass units 34.97 and 36.97. chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. The relative abundance of an isotope is 0.755 and. these differing atoms are called isotopes. The relative abundance of an isotope is 0.755 and. chlorine has two stable isotopes, 35 cl and 37. Chlorine Has Two Isotopes.
From www.numerade.com
SOLVEDChlorine has two natural isotopes 17^37 Cl and 17^35 Cl Chlorine Has Two Isotopes (a) when a sample of elemental chlorine is injected into. chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. There are two principal stable. all atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. chlorine has two isotopes, cl35 and cl37; All atoms of chlorine. Chlorine Has Two Isotopes.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram Chlorine Has Two Isotopes This is because chlorine contains two. chlorine has two isotopes, cl35 and cl37; The atomic weight of chlorine given on the periodic table is 35.47 u. chlorine has two stable isotopes: There are two principal stable. the mass spectrum of chlorine. The chlorine has multiple isotopes and is hit with a. chlorine has two stable isotopes,. Chlorine Has Two Isotopes.
From askfilo.com
Chlorine has two isotopes of aotmic mass unit 34.97u and 36.97u. The rela.. Chlorine Has Two Isotopes chlorine has two isotopes of atomic mass units 34.97 and 36.97. find the average atomic mass of chlorine. chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. all atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. The atomic weight of chlorine given on. Chlorine Has Two Isotopes.
From www.slideshare.net
Atomic Structure Part 2 Chlorine Has Two Isotopes Unlike protons, the number of neutrons is not absolutely fixed for most elements. The atomic mass of cl35 is 34.969. chlorine has two isotopes of atomic mass units 34.97 and 36.97. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. chlorine has 24 isotopes with mass numbers ranging from 28 cl. Chlorine Has Two Isotopes.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Has Two Isotopes 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine has two isotopes of atomic mass units 34.97 and 36.97. There are only two stable isotopes: chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. There are. Chlorine Has Two Isotopes.
From www.numerade.com
SOLVEDChlorine has two natural isotopes 17^37 Cl and 17^35 Cl Chlorine Has Two Isotopes these differing atoms are called isotopes. There are two principal stable. Chlorine has two isotopes of atomic mass units 34.97 and 36.97. Unlike protons, the number of neutrons is not absolutely fixed for most elements. The chlorine has multiple isotopes and is hit with a. All atoms of chlorine (cl) have 17 protons, but there are chlorine. chlorine. Chlorine Has Two Isotopes.
From www.pdffiller.com
Fillable Online The two naturally occurred isotopes of chlorine has Chlorine Has Two Isotopes The atomic mass of cl35 is 34.969. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51. Chlorine Has Two Isotopes.
From byjus.com
40. Atomic weight of chlorine is 35.5.It has two isotopes of atomic Chlorine Has Two Isotopes chlorine has two isotopes, cl35 and cl37; these differing atoms are called isotopes. The relative abundance of an isotope is 0.755 and. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. the mass spectrum of chlorine. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1. Chlorine Has Two Isotopes.
From www.youtube.com
Chlorine has two isotopes Cl35 and Cl37. Ratio present in nature is 31 Chlorine Has Two Isotopes Unlike protons, the number of neutrons is not absolutely fixed for most elements. chlorine has two isotopes of atomic mass units 34.97 and 36.97. the mass spectrum of chlorine. Chlorine is taken as typical of elements with more than one atom per molecule. chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to. Chlorine Has Two Isotopes.
From www.toppr.com
Chlorine has two naturally occurring isotopes, ^35Cl and ^37Cl . If the Chlorine Has Two Isotopes The relative abundances of these two isotopes. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The atomic weight of chlorine given on the periodic table is 35.47 u. chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. These are. Chlorine Has Two Isotopes.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass Chlorine Has Two Isotopes Chlorine is taken as typical of elements with more than one atom per molecule. The atomic mass of cl35 is 34.969. these differing atoms are called isotopes. These are its only two natural isotopes occurring in quantity, with 35 cl making up 76% of natural chlorine. chlorine has two stable isotopes: chlorine (cl) has isotopes with mass. Chlorine Has Two Isotopes.
From www.numerade.com
Title Isotopes of Chlorine and Average Atomic Mass Calculation While Chlorine Has Two Isotopes find the average atomic mass of chlorine. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other. chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. Chlorine has two stable isotopes: The relative abundances of these two isotopes. all atoms. Chlorine Has Two Isotopes.
From www.coursehero.com
[Solved] 13) A sample of chlorine has two naturally occurring isotopes Chlorine Has Two Isotopes Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other. find the average atomic mass of chlorine. All atoms of chlorine (cl) have 17 protons, but there are chlorine. the mass spectrum of chlorine. all atoms of chlorine (cl) have 17 protons, but there. Chlorine Has Two Isotopes.
From dxooqyxds.blob.core.windows.net
Chlorine Isotope Compositions at Irene Hart blog Chlorine Has Two Isotopes These are its only two natural isotopes occurring in quantity, with 35 cl making up 76% of natural chlorine. chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. these differing atoms are called isotopes. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass. Chlorine Has Two Isotopes.
From askfilo.com
Average atomic mass of chlorine is 35.5u. It has two isotopes of atomic m.. Chlorine Has Two Isotopes Chlorine has two stable isotopes: There are only two stable isotopes: This is because chlorine contains two. chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. We'll look at its mass spectrum to show. chlorine has two stable isotopes, 35 cl and 37 cl. There are two principal stable. for example, the relative. Chlorine Has Two Isotopes.
From www.toppr.com
17^35Cl and 17^37Cl are two isotopes of chlorine. If average atomic Chlorine Has Two Isotopes Chlorine has two isotopes of atomic mass units 34.97 and 36.97. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. these differing atoms are called isotopes. chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. chlorine has 24 isotopes with mass. Chlorine Has Two Isotopes.
From www.numerade.com
SOLVEDChlorine has two natural isotopes _{17}^{… Chlorine Has Two Isotopes This is because chlorine contains two. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. (a) when a sample of elemental chlorine is injected into. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. find the average atomic mass of chlorine. There are two principal. Chlorine Has Two Isotopes.
From kunduz.com
[ANSWERED] Chlorine atom has two naturally occurring isotopes 35Cl and Chlorine Has Two Isotopes in figure 2.3.8 you can see chlorine gas entering an mass spectrometer. find the average atomic mass of chlorine. these differing atoms are called isotopes. There are two principal stable. chlorine has two stable isotopes, 35 cl and 37 cl. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio.. Chlorine Has Two Isotopes.
From brainly.com
Chlorine has two isotopes, 35Cl and 37Cl; 75.77 of chlorine is 35Cl Chlorine Has Two Isotopes These are its only two natural isotopes occurring in quantity, with 35 cl making up 76% of natural chlorine. the mass spectrum of chlorine. We'll look at its mass spectrum to show. Chlorine has two stable isotopes: The atomic weight of chlorine given on the periodic table is 35.47 u. chlorine (cl) has isotopes with mass numbers ranging. Chlorine Has Two Isotopes.
From scienceprism.co.uk
How archaeologists use chemistry to find out about our past Science Prism Chlorine Has Two Isotopes Chlorine is taken as typical of elements with more than one atom per molecule. We'll look at its mass spectrum to show. find the average atomic mass of chlorine. chlorine has two isotopes of atomic mass units 34.97 and 36.97. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks. these. Chlorine Has Two Isotopes.
From www.coursehero.com
[Solved] Chlorine has two naturally occurring isotopes Chlorine 35 Chlorine Has Two Isotopes chlorine has two stable isotopes, 35 cl and 37 cl. There are two principal stable. chlorine has two stable isotopes: the mass spectrum of chlorine. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. This is because chlorine contains two. Chlorine has two isotopes, with 75.53% being 35 cl with an. Chlorine Has Two Isotopes.
From www.chegg.com
Solved Q15. Chlorine has two isotopes Cl35 and Cl37. The Chlorine Has Two Isotopes 75.77% of chlorine is cl35, and 24.23% is cl37. the mass spectrum of chlorine. chlorine has two stable isotopes, 35 cl and 37 cl. all atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. these differing atoms are called isotopes. The relative abundance of an isotope is 0.755. Chlorine Has Two Isotopes.
From askfilo.com
Chlorine has two isotopes 35Cl and 37Cl. If the abundance of lighter is.. Chlorine Has Two Isotopes This is because chlorine contains two. The relative abundance of an isotope is 0.755 and. chlorine has two isotopes, cl35 and cl37; 75.77% of chlorine is cl35, and 24.23% is cl37. The relative abundance of an isotope is 0.755 and. All atoms of chlorine (cl) have 17 protons, but there are chlorine. The chlorine has multiple isotopes and is. Chlorine Has Two Isotopes.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6461 Science Chlorine Has Two Isotopes The relative abundance of an isotope is 0.755 and. these differing atoms are called isotopes. chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. chlorine has two isotopes, cl35 and cl37; naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of.. Chlorine Has Two Isotopes.
From www.doubtnut.com
Atomic weight of chlorine is 35.5 . It has two isotopes of atomic weig Chlorine Has Two Isotopes The natural abundance of these two isotopes is observed in the mass spectrum as two peaks. chlorine has two stable isotopes, 35 cl and 37 cl. find the average atomic mass of chlorine. The atomic weight of chlorine given on the periodic table is 35.47 u. These are its only two natural isotopes occurring in quantity, with 35. Chlorine Has Two Isotopes.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413991 Chlorine Has Two Isotopes chlorine has two isotopes of atomic mass units 34.97 u and 36.97 u. The relative abundances of these two isotopes. these differing atoms are called isotopes. chlorine has two isotopes of atomic mass units 34.97 and 36.97. chlorine has two stable isotopes: 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to. Chlorine Has Two Isotopes.
From www.toppr.com
The isotopes of chlorine with mass number 35 and 37 exist in the ratio of Chlorine Has Two Isotopes 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. All atoms of chlorine (cl) have 17 protons, but there are chlorine. chlorine has two isotopes of atomic mass units 34.97 and 36.97. chlorine has two isotopes of atomic mass units 34.97 u and. Chlorine Has Two Isotopes.
From www.alamy.com
Isotopes of chlorine. Illustration showing the two principal stable Chlorine Has Two Isotopes These are its only two natural isotopes occurring in quantity, with 35 cl making up 76% of natural chlorine. The relative abundance of an isotope is 0.755 and. There are only two stable isotopes: The atomic mass of cl35 is 34.969. find the average atomic mass of chlorine. naturally occurring chlorine consists of 35 cl (mass 34.96885 amu). Chlorine Has Two Isotopes.
From www.numerade.com
SOLVED Chlorine has two naturally occuring isotopes chlorine35 and Chlorine Has Two Isotopes There are only two stable isotopes: Chlorine is taken as typical of elements with more than one atom per molecule. These are its only two natural isotopes occurring in quantity, with 35 cl making up 76% of natural chlorine. All atoms of chlorine (cl) have 17 protons, but there are chlorine. Chlorine has two isotopes, with 75.53% being 35 cl. Chlorine Has Two Isotopes.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Has Two Isotopes These are its only two natural isotopes occurring in quantity, with 35 cl making up 76% of natural chlorine. The relative abundances of these two isotopes. There are two principal stable. find the average atomic mass of chlorine. We'll look at its mass spectrum to show. There are only two stable isotopes: for example, the relative atomic mass. Chlorine Has Two Isotopes.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Chlorine Has Two Isotopes chlorine has two stable isotopes, 35 cl and 37 cl. Unlike protons, the number of neutrons is not absolutely fixed for most elements. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other. for example, the relative atomic mass of chlorine is 35.5 rather than. Chlorine Has Two Isotopes.
From www.toppr.com
Chlorine has two isotopes of atomic mass units 34.97 and 36.97. The Chlorine Has Two Isotopes (a) when a sample of elemental chlorine is injected into. The atomic weight of chlorine given on the periodic table is 35.47 u. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. the mass spectrum of chlorine. Chlorine is taken as typical of elements with more than one atom per molecule. chlorine. Chlorine Has Two Isotopes.
From www.toppr.com
Chlorine has two isotopes of atomic mass units 34.97 and 36.97. The Chlorine Has Two Isotopes all atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. Chlorine is. Chlorine Has Two Isotopes.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Chlorine Has Two Isotopes 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are two principal stable. Chlorine has two isotopes of atomic mass units 34.97 and 36.97. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. chlorine has two. Chlorine Has Two Isotopes.