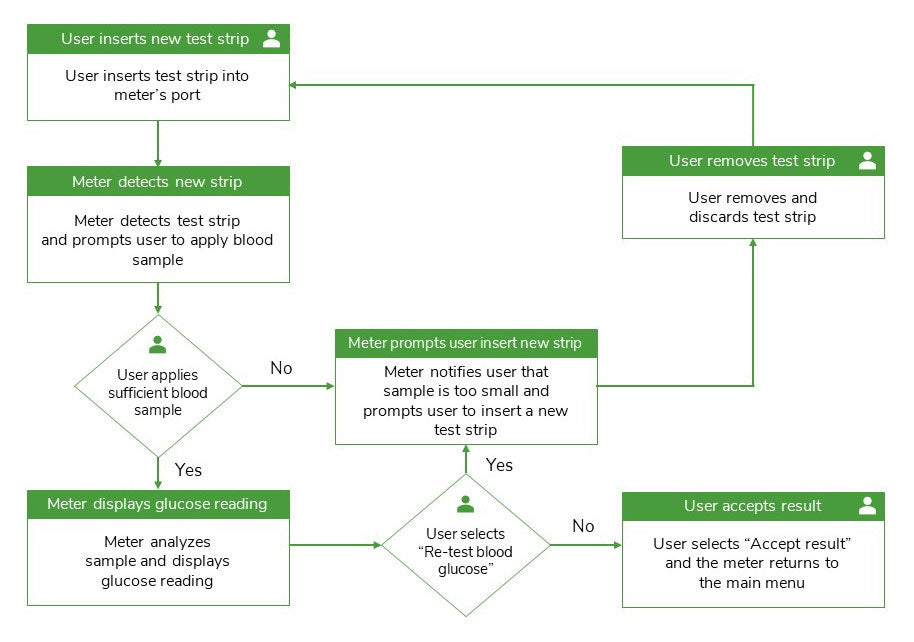
Medical Device Task Analysis . Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Comprehensive human factors analysis for medical device development. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda would like to see a clear statement on the. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“.
from www.emergobyul.com
The fda would like to see a clear statement on the. Comprehensive human factors analysis for medical device development. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the.
Medical Device Risk Analysis for Human Factors Emergo by UL
Medical Device Task Analysis The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Comprehensive human factors analysis for medical device development. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. The fda would like to see a clear statement on the. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file.
From www.researchgate.net
Standard surgical hierarchical task analysis of laparoscopic Medical Device Task Analysis The fda would like to see a clear statement on the. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. The. Medical Device Task Analysis.
From mungfali.com
Task Analysis Chart Medical Device Task Analysis Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. The fda would like to see a clear statement on the. Utilize our extensive human factors toolkit. Medical Device Task Analysis.
From www.researchgate.net
(PDF) An Extended Hierarchical Task Analysis for Error Prediction in Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. The fda would like to see a clear statement on the. Comprehensive human factors analysis for medical device development. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. Task analysis and risk analysis, safe medical devices, the summative evaluation. Medical Device Task Analysis.
From www.orielstat.com
Choosing the right medical device risk management tools Medical Device Task Analysis The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. The fda would like to see a clear statement on the. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical. Medical Device Task Analysis.
From www.aligned.ch
Task Analysis as Requirement Management method in Medical Device Medical Device Task Analysis The fda would like to see a clear statement on the. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis. Medical Device Task Analysis.
From www.slideserve.com
PPT Task Analysis PowerPoint Presentation, free download ID5716470 Medical Device Task Analysis The fda would like to see a clear statement on the. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. The. Medical Device Task Analysis.
From shotnelo.weebly.com
Medical device risk assessment template shotnelo Medical Device Task Analysis Fda has developed this guidance document to assist industry in following appropriate human factors and usability. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Comprehensive human factors analysis for medical device development. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Task analysis. Medical Device Task Analysis.
From www.researchgate.net
Hierarchical task analysis of laparoscopic right colectomy Download Table Medical Device Task Analysis Comprehensive human factors analysis for medical device development. The fda would like to see a clear statement on the. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant. Medical Device Task Analysis.
From wbrlabs.in
Medical device Biological Evaluation [ISO 10993] WBR LABS Medical Device Task Analysis The fda would like to see a clear statement on the. Comprehensive human factors analysis for medical device development. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Utilize our extensive. Medical Device Task Analysis.
From americantemplates.com
18+ Free Task Analysis Examples & Templates Sample PDF » American Medical Device Task Analysis Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. The fda would like to see. Medical Device Task Analysis.
From www.ihf.co.uk
How to conduct a safety critical task analysis IHF Ltd Medical Device Task Analysis Fda has developed this guidance document to assist industry in following appropriate human factors and usability. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. The fda now defines “tasks”. Medical Device Task Analysis.
From www.greenlight.guru
What Device Makers Need To Know About Design Verification and Validation Medical Device Task Analysis The fda would like to see a clear statement on the. Comprehensive human factors analysis for medical device development. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Utilize our extensive. Medical Device Task Analysis.
From data1.skinnyms.com
Medical Device Traceability Matrix Template Medical Device Task Analysis Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Comprehensive human factors analysis for medical device development. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda would like to see a clear statement on the. Utilize our extensive human factors toolkit to. Medical Device Task Analysis.
From www.sampletemplates.com
FREE 15+ Task Analysis Samples in PDF Excel MS Word Google Docs Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Comprehensive human factors analysis for medical device development.. Medical Device Task Analysis.
From www.greenlight.guru
Design Controls For Medical Device Companies [Guide] Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. The fda would like to see a clear statement on the. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired. Medical Device Task Analysis.
From www.researchgate.net
High level task analysis for all the devices under evaluation Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. The fda would like to see a clear statement on the. Comprehensive human factors analysis for medical device development. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda now defines “tasks” as “one or more user interactions. Medical Device Task Analysis.
From www.modernrequirements.com
Facilitate the Medical Device Design Controls Modern Requirements Medical Device Task Analysis Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. The fda would like to see a clear statement on the. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. Comprehensive. Medical Device Task Analysis.
From www.crossrivertherapy.com
Task Analysis In ABA Therapy Examples & Strategies Medical Device Task Analysis The fda would like to see a clear statement on the. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Comprehensive human factors analysis for medical device development. Utilize our extensive. Medical Device Task Analysis.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. The fda would like to see a clear statement on the. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. In. Medical Device Task Analysis.
From masoprealty.weebly.com
Medical device risk assessment template masoprealty Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda would like to see a clear statement on the. Comprehensive. Medical Device Task Analysis.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Task Analysis Comprehensive human factors analysis for medical device development. The fda would like to see a clear statement on the. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Fda has developed this guidance document to. Medical Device Task Analysis.
From americantemplates.com
18+ Free Task Analysis Examples & Templates Sample PDF » American Medical Device Task Analysis The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Comprehensive human factors analysis for medical device development. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Utilize our extensive human factors toolkit to. Medical Device Task Analysis.
From sunstonepilot.com
What Are Medical Device Design Controls? Sunstone Pilot, Inc. Medical Device Task Analysis Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Comprehensive human factors analysis for medical device development. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. The fda would like to see a clear statement. Medical Device Task Analysis.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. The fda would like to see a clear statement on the. Comprehensive human factors analysis for medical device development. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Task analysis. Medical Device Task Analysis.
From americantemplates.com
18+ Free Task Analysis Examples & Templates Sample PDF » American Medical Device Task Analysis Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. The. Medical Device Task Analysis.
From community.greenlight.guru
Overview of Medical Device clinical activities updated table from ISO Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Comprehensive human factors analysis for medical device development. The fda would like to see a clear statement on the. The fda. Medical Device Task Analysis.
From www.springboard.pro
Design controls in medical device development Ensuring safety and Medical Device Task Analysis The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Task. Medical Device Task Analysis.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Task Analysis Fda has developed this guidance document to assist industry in following appropriate human factors and usability. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Comprehensive human factors analysis for medical. Medical Device Task Analysis.
From medicaldeviceacademy.com
URRA table example from the FDA Medical Device Academy Medical Device Task Analysis Comprehensive human factors analysis for medical device development. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. The fda. Medical Device Task Analysis.
From demigos.com
A Guide to Medical Image Analysis Software Development Medical Device Task Analysis The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. The fda would like to see a clear statement on the. Comprehensive human factors analysis for medical device development. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Utilize our extensive. Medical Device Task Analysis.
From www.mdpi.com
Healthcare Free FullText Optimal Usability Test Procedure Medical Device Task Analysis In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Comprehensive human factors. Medical Device Task Analysis.
From www.emergobyul.com
Medical Device Risk Analysis for Human Factors Emergo by UL Medical Device Task Analysis Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. The fda would like to see. Medical Device Task Analysis.
From creanova.com
Usability Engineering in Medical Devices industry Creanova Medical Device Task Analysis The fda would like to see a clear statement on the. Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda now defines “tasks” as “one or more user interactions with a medical device to achieve a desired result“. Comprehensive human factors analysis for medical device development. Utilize our extensive human factors. Medical Device Task Analysis.
From www.scribd.com
Medical Device Design Verification SOP Medical Device Task Analysis Utilize our extensive human factors toolkit to enhance cognitive and physical interactions, supporting the. The fda would like to see a clear statement on the. Comprehensive human factors analysis for medical device development. Task analysis and risk analysis, safe medical devices, the summative evaluation of medical devices, a compliant usability file. Fda has developed this guidance document to assist industry. Medical Device Task Analysis.
From www.collidu.com
Software as a Medical Device (SaMD) PowerPoint and Google Slides Medical Device Task Analysis Fda has developed this guidance document to assist industry in following appropriate human factors and usability. The fda would like to see a clear statement on the. In section 3.1, we describe preliminary hazard analysis for medical devices, provide a task analysis in a risk management process for usability testing and examine. Comprehensive human factors analysis for medical device development.. Medical Device Task Analysis.