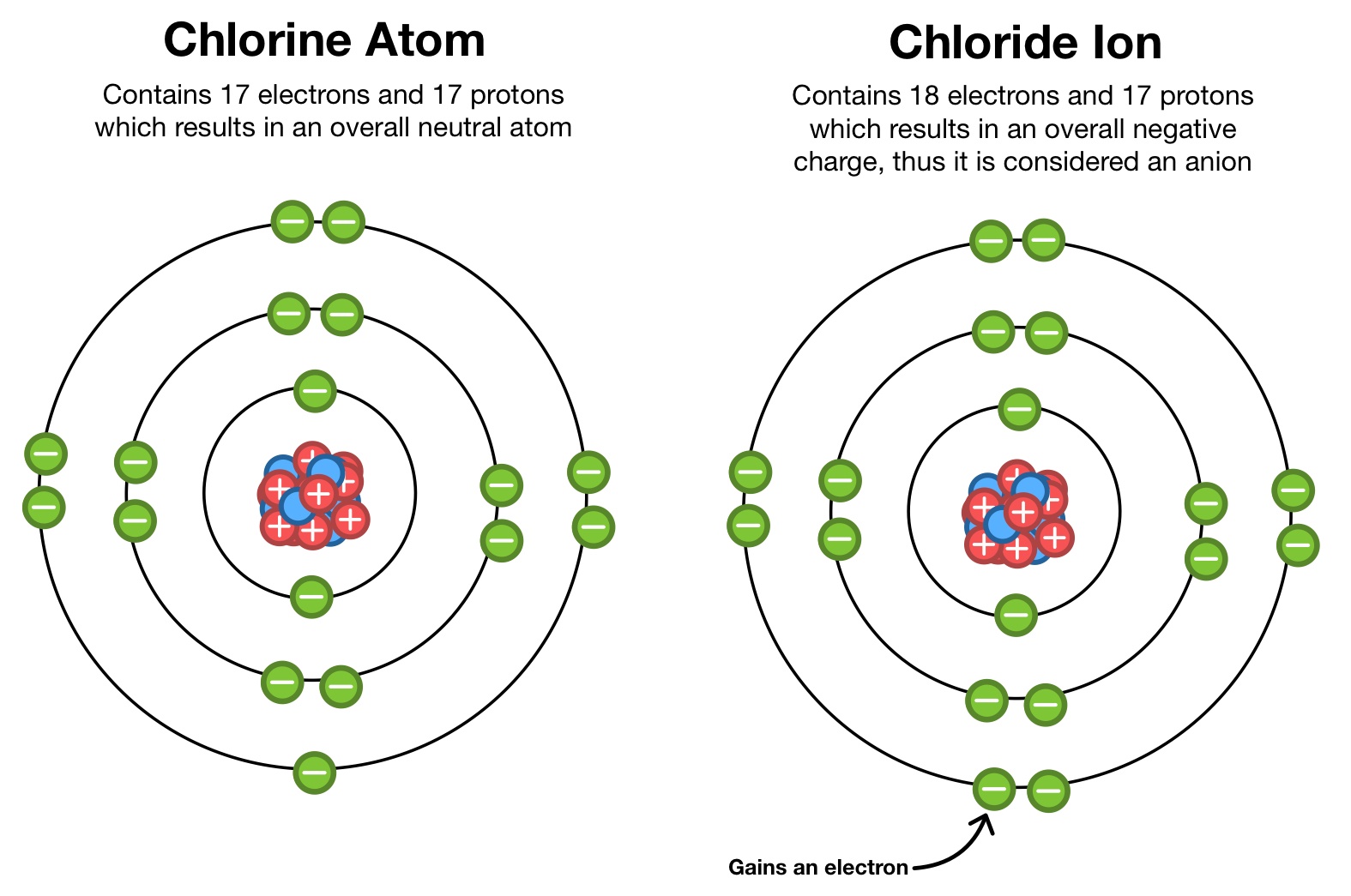
Chlorine Cation . Chloride already specifically means the ion clx− c l x −. Due to their opposite charges, they attract each other to form an ionic lattice. If chlorine was to try and remove the electron from. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. That is such an odd. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion.
from dxopaibhs.blob.core.windows.net
Chloride already specifically means the ion clx− c l x −. If chlorine was to try and remove the electron from. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. That is such an odd. Due to their opposite charges, they attract each other to form an ionic lattice. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom.
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at
Chlorine Cation If chlorine was to try and remove the electron from. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. Chloride already specifically means the ion clx− c l x −. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. That is such an odd. Due to their opposite charges, they attract each other to form an ionic lattice. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. If chlorine was to try and remove the electron from.
From www.researchgate.net
The low energy end of the LIF spectrum of chlorine cation. The top Chlorine Cation // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. If chlorine was to try and remove the electron from. Chloride already specifically means the ion clx− c l x −. Due to their opposite charges, they attract each other to form an ionic lattice. A cation (a positive ion) forms when a neutral. Chlorine Cation.
From www.dreamstime.com
Diagram Representation of the Element Chlorine Stock Illustration Chlorine Cation // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Chloride already specifically means the ion clx− c l x −. Consequently hydrogen does not form. Chlorine Cation.
From periodictableguide.com
Chlorine (Cl) Periodic Table (Element Information & More) Chlorine Cation For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. If chlorine was to try and remove the electron from. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. A cation (a positive ion) forms when a neutral atom. Chlorine Cation.
From blog.orendatech.com
Does chlorine affect pH? Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Chloride already specifically means the ion clx− c l x −. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom.. Chlorine Cation.
From www.schoolmouv.fr
Les ions cours 5e (et 4e, 3e) Physiquechimie SchoolMouv Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. Consequently hydrogen does not form a cation, but can result in an acid, which is a. Chlorine Cation.
From www.verifiedmarketresearch.com
Chlorine Gas Detector Market Size, Scope, Growth, Trends Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Due to their opposite charges, they attract each other to form an ionic lattice. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent. Chlorine Cation.
From www.aquaportail.com
Cation définition et explications Chlorine Cation Due to their opposite charges, they attract each other to form an ionic lattice. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. Chloride already specifically means the ion clx− c l x −. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion.. Chlorine Cation.
From www.numerade.com
SOLVED most likely ion element symbol of ion type of ion 3 + Sc Chlorine Cation For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Chloride already specifically means the ion clx− c l x −.. Chlorine Cation.
From www.getbodysmart.com
Ions Cations and Anions GetBodySmart Chlorine Cation Due to their opposite charges, they attract each other to form an ionic lattice. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. If chlorine was to try and remove the electron from. That is such an odd. For example, in. Chlorine Cation.
From www.numerade.com
SOLVED most likely ion element symbol of ion type of ion scandium Sc3 Chlorine Cation For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. Chloride already specifically means the ion clx− c l x −. Consequently hydrogen does not form a cation, but can result in an. Chlorine Cation.
From www.shutterstock.com
Anions Cations Example Sodium Chlorine Atoms ilustrações stock Chlorine Cation // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. That is such an odd. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. Due to their opposite charges, they attract each other to form an ionic lattice. A cation (a positive ion) forms. Chlorine Cation.
From www.gwpumps.com
Chlorination System GW Pumps Chlorine Cation Due to their opposite charges, they attract each other to form an ionic lattice. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. If chlorine was to try and remove the electron from. That is such an odd. A cation (a positive ion) forms when a neutral atom. Chlorine Cation.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Chloride already specifically means the ion clx− c l x −. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. // for. Chlorine Cation.
From www.researchgate.net
The low energy end of the LIF spectrum of chlorine cation. The top Chlorine Cation Chloride already specifically means the ion clx− c l x −. Due to their opposite charges, they attract each other to form an ionic lattice. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Consequently hydrogen does not form a cation,. Chlorine Cation.
From cartoondealer.com
Anions And Cations For Example Sodium And Chlorine Atoms. Cartoon Chlorine Cation Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. That is such an odd. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. If chlorine was to try and remove the. Chlorine Cation.
From stagesc10.watts.ae
Chlorine Micro Check Test Strips HF scientific Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. If chlorine was to try and remove the electron from. That is such an odd. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar. Chlorine Cation.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Due to their opposite charges, they attract each other to form an ionic lattice. If chlorine was to try and remove the electron from. A cation (a positive ion) forms when a. Chlorine Cation.
From www.aquaportail.com
Oxyanion définition et explications Chlorine Cation Due to their opposite charges, they attract each other to form an ionic lattice. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. That is such an odd. A cation (a positive ion) forms. Chlorine Cation.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Chloride already specifically means the ion clx− c l x −. That is such an odd. If chlorine was to try and remove the electron from. Due to their opposite charges, they. Chlorine Cation.
From www.kentwater.ca
The Importance of Chlorine/Chloramine Removal Water Filters Kent Chlorine Cation // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one. Chlorine Cation.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Chloride already specifically means the ion clx− c l x −. Due to their opposite charges, they attract each other to form an ionic lattice. If chlorine was to try and remove. Chlorine Cation.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Cation // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. Chloride already specifically means. Chlorine Cation.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Cation A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. If chlorine was to try and remove the electron from. That is such an odd. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron. Chlorine Cation.
From www.chegg.com
Solved Complete the table below. For example, in the first Chlorine Cation Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. That is such an odd. Due to their opposite charges, they attract each other to form an ionic lattice. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. Chloride already specifically means the ion. Chlorine Cation.
From butchixanh.edu.vn
Common Anions List and Formulas Bút Chì Xanh Chlorine Cation For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. That is such an odd. Chloride already specifically means the ion clx− c l x −. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a. Chlorine Cation.
From www.greelane.com
Où trouveton le chlore dans le tableau périodique Chlorine Cation Due to their opposite charges, they attract each other to form an ionic lattice. That is such an odd. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Consequently hydrogen does not form a cation, but can result in an acid,. Chlorine Cation.
From www.youtube.com
DSE02V00Formation of cation and anion YouTube Chlorine Cation For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. That is such an odd. Due to their opposite charges, they attract each other to form an ionic lattice. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. If. Chlorine Cation.
From www.chegg.com
Solved most likely ion element symbol of ion type of ion Chlorine Cation Due to their opposite charges, they attract each other to form an ionic lattice. Chloride already specifically means the ion clx− c l x −. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell,. Chlorine Cation.
From www.numerade.com
SOLVED 'This image shows............... This image shows points 37 1 Chlorine Cation Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. That is such an odd. If chlorine was to try and remove the electron from. Due to their opposite charges, they attract each other to. Chlorine Cation.
From jeopardylabs.com
Unit 1 Test Jeopardy Template Chlorine Cation If chlorine was to try and remove the electron from. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. Due to their opposite charges, they attract each other to form an ionic lattice. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na. Chlorine Cation.
From www.wikidoc.org
Chlorine wikidoc Chlorine Cation Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. That is such an odd. Chloride already specifically means the ion clx− c. Chlorine Cation.
From www.researchgate.net
The low energy end of the LIF spectrum of chlorine cation. The top Chlorine Cation If chlorine was to try and remove the electron from. Due to their opposite charges, they attract each other to form an ionic lattice. That is such an odd. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Chloride already specifically. Chlorine Cation.
From www.britannica.com
Chlorine Uses, Properties, & Facts Britannica Chlorine Cation Chloride already specifically means the ion clx− c l x −. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Due to their opposite charges, they attract each other to form an ionic lattice. If chlorine was to try and remove. Chlorine Cation.
From blog.techrev.us
In The Swim 3 Inch Stabilized Chlorine Tablets for Sanitizing Swimming Chlorine Cation Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. Due to their opposite charges, they attract each other to form an ionic lattice. // for clx+ c l x + is use chlorine (i) cation or chlorine (1+) ion. For example, in the reaction of na (sodium) and cl (chlorine),. Chlorine Cation.
From www.but.fr
Chlore Choc Pastille 5kg Chloriklar Entretien Equipement de la Chlorine Cation Chloride already specifically means the ion clx− c l x −. If chlorine was to try and remove the electron from. Consequently hydrogen does not form a cation, but can result in an acid, which is a polar covalent bond. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and. Chlorine Cation.