Calorimetry Lab Report Prezi . This value is the final calorimeter. 6.03 calorimetry lab report by; Determining the specific heat of an unknown metal. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. 6.03 calorimetry lab report by: 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. It defines enthalpy change as the heat transfer of a system when. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry.
from www.studocu.com
A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. It defines enthalpy change as the heat transfer of a system when. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. This value is the final calorimeter. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. 6.03 calorimetry lab report by; This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Determining the specific heat of an unknown metal. 6.03 calorimetry lab report by:
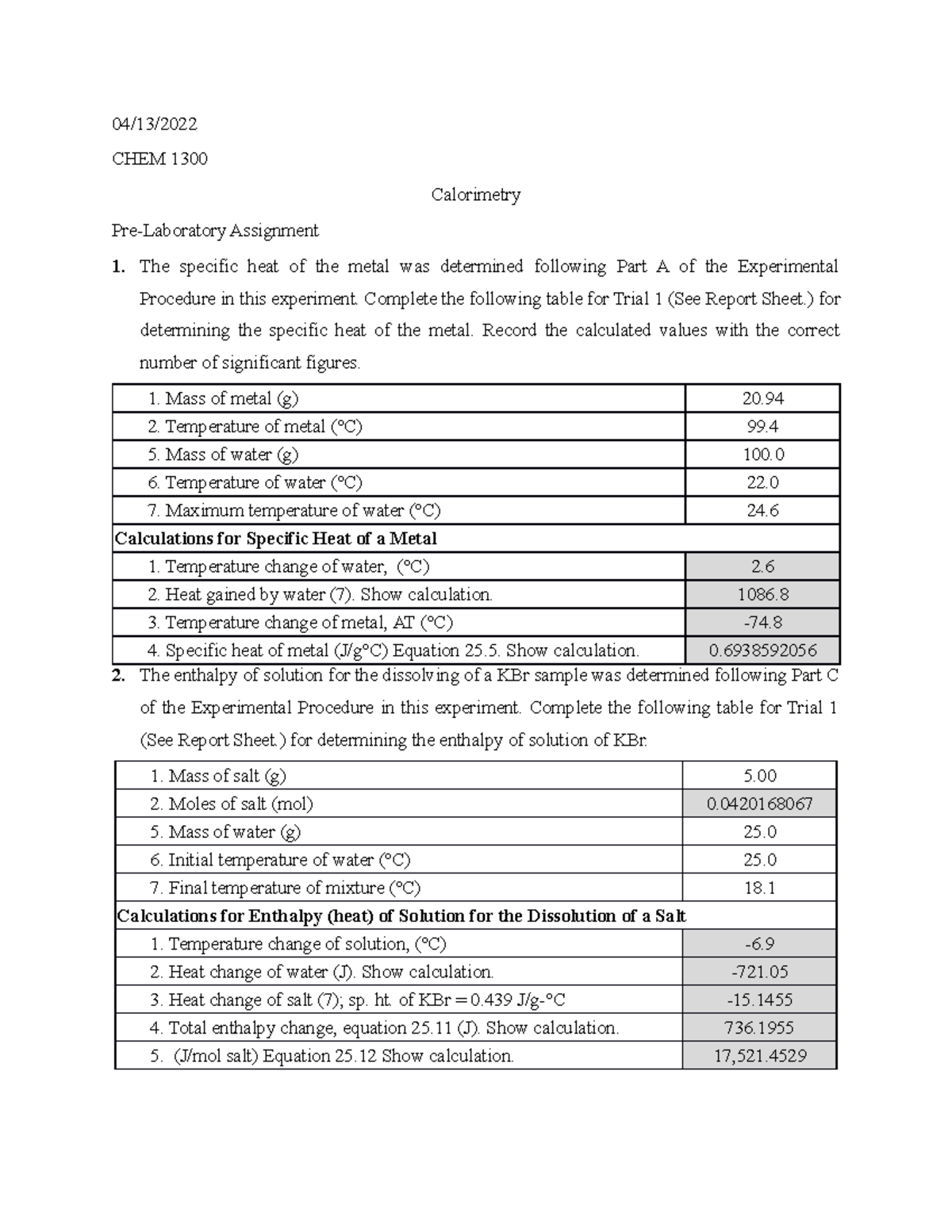
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory
Calorimetry Lab Report Prezi Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. It defines enthalpy change as the heat transfer of a system when. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. 6.03 calorimetry lab report by: This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. 6.03 calorimetry lab report by; 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. Determining the specific heat of an unknown metal. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. This value is the final calorimeter.
From www.studocu.com
Calorimetry Lab Report Calorimetry Lab Report Introduction Calorimetry Lab Report Prezi Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. Determining the specific heat of an unknown metal. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the. Calorimetry Lab Report Prezi.
From browsegrades.net
Lab Report >7.03 Calorimetry Lab Report_ Parkersburg South High School Calorimetry Lab Report Prezi Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. Determining the specific heat of an unknown metal. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat =. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Report Physics 212 General Physics With Calculus 212 Calorimetry Lab Report Prezi 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. It defines enthalpy change. Calorimetry Lab Report Prezi.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Calorimetry Lab Report Prezi 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. 6.03 calorimetry lab report by: 1.place a plastic measuring trough on top of the digital balance, and press. Calorimetry Lab Report Prezi.
From www.chegg.com
Solved CALORIMETRY HEAT CAPACITY OF A CALORIMETER Calorimetry Lab Report Prezi It defines enthalpy change as the heat transfer of a system when. 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. Abstract the main purpose of the calorimetry. Calorimetry Lab Report Prezi.
From www.studocu.com
Lab Report Calorimetry Lab Report CALORIMETRY Introduction The Calorimetry Lab Report Prezi This value is the final calorimeter. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. 6.03 calorimetry lab report by: 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. 1 calorimetry lab alejandra beta,. Calorimetry Lab Report Prezi.
From www.studocu.com
Online Calorimetry Labs Calorimetry Lab Report Sheet Name Quiz is Calorimetry Lab Report Prezi 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. Determining the specific heat of an unknown metal. 6.03 calorimetry lab report by: Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. It defines enthalpy change as the heat transfer of. Calorimetry Lab Report Prezi.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Calorimetry Lab Report Prezi 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. Determining the specific heat of an unknown metal. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at. Calorimetry Lab Report Prezi.
From www.scribd.com
Pre Lab Report Calorimetry PDF Calorimetry Lab Report Prezi This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. It defines enthalpy change as the heat transfer of a system when. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. Determining the specific heat of an unknown metal. Solve the two linear equations obtained from the graph. Calorimetry Lab Report Prezi.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Report Prezi This value is the final calorimeter. Determining the specific heat of an unknown metal. It defines enthalpy change as the heat transfer of a system when. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry.. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Report (Part 1) For this experiment, the definition Calorimetry Lab Report Prezi Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. Determining the specific heat of an unknown metal. Solve the two linear equations obtained from the graph tangents. Calorimetry Lab Report Prezi.
From www.studocu.com
UTF8''Prelabbomb calorimetry Chemistry 122 Laboratory Bomb Calorimetry Lab Report Prezi Determining the specific heat of an unknown metal. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. 1.place a plastic measuring trough on top of the digital balance,. Calorimetry Lab Report Prezi.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Kim Smith Lab Calorimetry Lab Report Prezi Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. 6.03 calorimetry lab report by; 6.03 calorimetry lab report by: 1.place a plastic measuring trough on top of. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Calorimetry Lab Report Prezi A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1.. Calorimetry Lab Report Prezi.
From www.studocu.com
7. Calorimetry Lab COPY Experiment 6. (1 week) (LCA) Calorimetry Calorimetry Lab Report Prezi Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. It defines enthalpy change as the heat transfer of a system when. This value is the final calorimeter. A calorimeter is used to measure changes in thermodynamic quantities and is composed. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Report Prezi Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. Determining the specific. Calorimetry Lab Report Prezi.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Calorimetry Lab Report Prezi 6.03 calorimetry lab report by; 6.03 calorimetry lab report by: This value is the final calorimeter. It defines enthalpy change as the heat transfer of a system when. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. Selina pfuner calculations. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Lab Report Prezi A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. It defines enthalpy change as the heat transfer of a system when. Abstract the main purpose of the calorimetry experiment is to measure. Calorimetry Lab Report Prezi.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Calorimetry Lab Report Prezi 6.03 calorimetry lab report by: This value is the final calorimeter. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. Determining the specific heat of an unknown metal. 6.03 calorimetry lab report by; 1 calorimetry lab alejandra beta, #20 2 purpose. Calorimetry Lab Report Prezi.
From www.studocu.com
Pre lab 3 pre lab report on identifying enthalpy of reaction using Calorimetry Lab Report Prezi 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. Determining the specific heat of an unknown metal. Selina pfuner calculations p2 unknown metals q[water]= m x c x. Calorimetry Lab Report Prezi.
From studylib.net
Student instructions and lab report Calorimetry Lab Report Prezi Determining the specific heat of an unknown metal. 6.03 calorimetry lab report by; 6.03 calorimetry lab report by: Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. It defines enthalpy change as the heat transfer of a system when. 1.place a plastic measuring trough on top of the digital balance, and. Calorimetry Lab Report Prezi.
From pdfprof.com
experiment 25 calorimetry lab report Calorimetry Lab Report Prezi 6.03 calorimetry lab report by: This value is the final calorimeter. Determining the specific heat of an unknown metal. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Solve the two linear equations obtained from the graph tangents simultaneously to. Calorimetry Lab Report Prezi.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry Lab Report Prezi Determining the specific heat of an unknown metal. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. 6.03 calorimetry lab. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Report S Calorimetry Introduction In this experiment Calorimetry Lab Report Prezi Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value. Calorimetry Lab Report Prezi.
From www.studypool.com
SOLUTION Calorimetry Lab Studypool Calorimetry Lab Report Prezi 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. Determining the specific heat of an unknown metal. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. 6.03 calorimetry lab report by; 6.03 calorimetry lab report by: It defines enthalpy change as the heat. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Report Experiment 6. (1 week) (LCA) Calorimetry Calorimetry Lab Report Prezi 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m. Calorimetry Lab Report Prezi.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Report Prezi 6.03 calorimetry lab report by; Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. This value is the final calorimeter. Selina pfuner calculations p2 unknown metals q[water]= m x c x. Calorimetry Lab Report Prezi.
From studylib.net
06.03 Calorimetry Lab Report Calorimetry Lab Report Prezi Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. It defines enthalpy change as the heat transfer of a system when. A calorimeter is used to measure changes in thermodynamic quantities and is composed. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Report Experiment 6. Calorimetry Purpose The purpose Calorimetry Lab Report Prezi This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. 6.03 calorimetry lab report by: Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. 6.03 calorimetry lab report by; 1. Calorimetry Lab Report Prezi.
From www.scribd.com
Calorimetry Lab Report PDF Calorie Water Calorimetry Lab Report Prezi It defines enthalpy change as the heat transfer of a system when. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container. This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Selina pfuner. Calorimetry Lab Report Prezi.
From www.studocu.com
CoffeeCup Calorimetry Experiment Lab Report with answers and solutions Calorimetry Lab Report Prezi Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. Selina pfuner calculations p2 unknown metals q[water]= m x c x deltat m = 24.5 g c=4.18 deltat = 29.1. A calorimeter is used to measure changes in thermodynamic quantities and is composed of an insulated container.. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Experiment 6. (1 week) (LCA) Calorimetry Purpose To Calorimetry Lab Report Prezi This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. 1.place a plastic measuring trough on top of the digital balance, and press the tare/on. 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. Determining the specific heat of an unknown metal. Selina pfuner. Calorimetry Lab Report Prezi.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Report Prezi 6.03 calorimetry lab report by: This document discusses measuring and expressing enthalpy changes (δh) through calorimetry. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. A calorimeter is. Calorimetry Lab Report Prezi.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Lab Report Prezi Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. It defines enthalpy change as the heat transfer of a system when. Determining the specific heat of an unknown metal. This value is the final calorimeter. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value. Calorimetry Lab Report Prezi.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Lab Report Prezi 1 calorimetry lab alejandra beta, #20 2 purpose the purpose of this lab was to measure the change in temperature, and then use that. Solve the two linear equations obtained from the graph tangents simultaneously to obtain the temperature value at which the two tangent lines cross. It defines enthalpy change as the heat transfer of a system when. A. Calorimetry Lab Report Prezi.