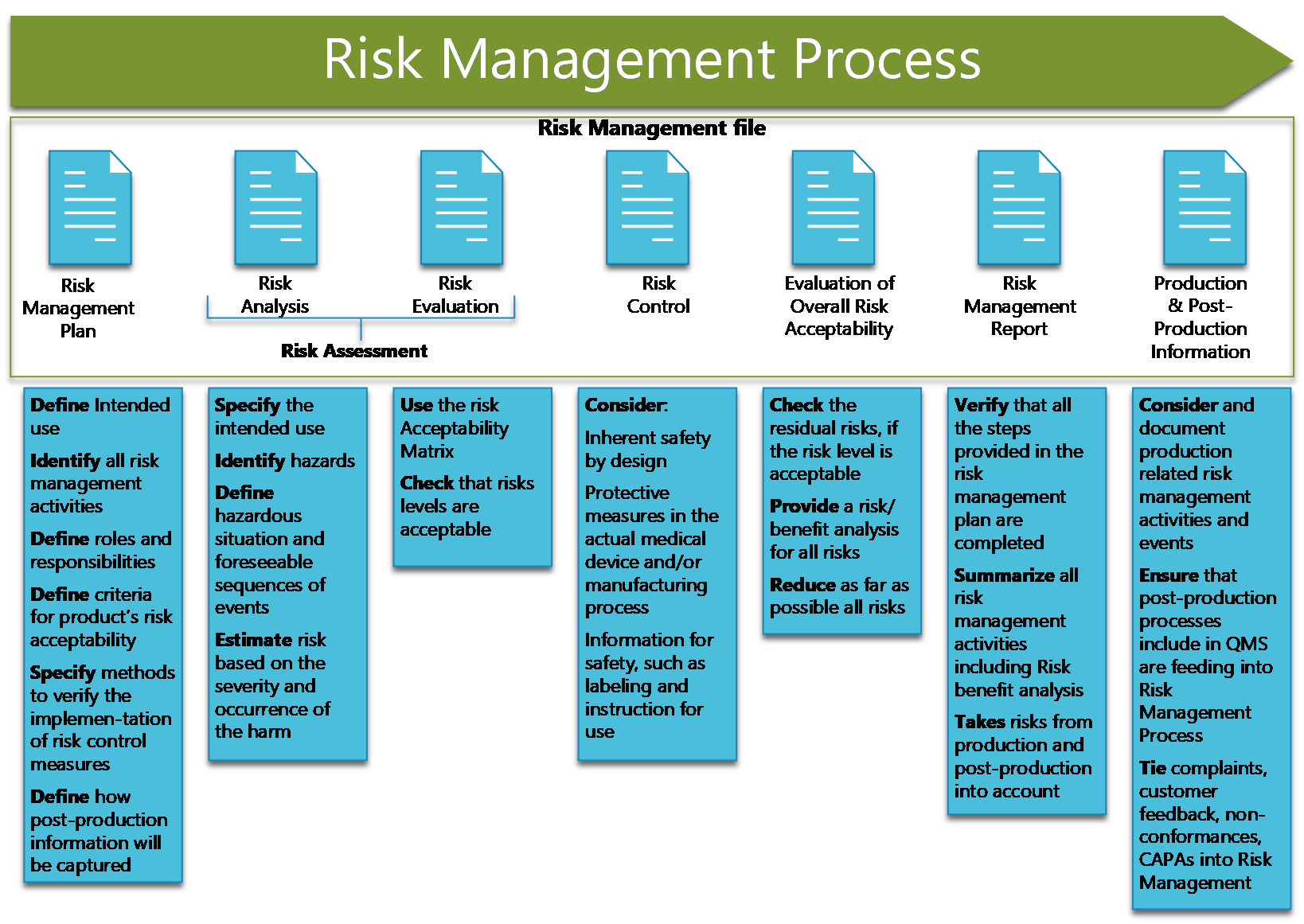
Device Risk Management Plan . Discuss the reasons for conducting risk management activities for medical devices. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Identify when to use risk management. Covering all elements of the risk management process. Further, the plan needs to establish how the device risks will be Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Rmp aims for the risks of drugs to be evaluated at.
from kvalito.ch
Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk management. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Rmp aims for the risks of drugs to be evaluated at. Covering all elements of the risk management process. Further, the plan needs to establish how the device risks will be Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles.
Risk Management for Medical Devices ISO 149712019 Kvalito
Device Risk Management Plan Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Rmp aims for the risks of drugs to be evaluated at. Identify when to use risk management. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Covering all elements of the risk management process. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Further, the plan needs to establish how the device risks will be Discuss the reasons for conducting risk management activities for medical devices.
From blog.clevercompliance.io
What is Risk Management and a Medical Device Risk Management Plan? Device Risk Management Plan This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Identify when to use risk management. Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Discuss the reasons for conducting risk management activities for medical devices. Rmp. Device Risk Management Plan.
From matrixreq.com
What is ISO 14971 Risk Management for Medical Devices? Device Risk Management Plan Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Identify when to. Device Risk Management Plan.
From www.mpo-mag.com
EMC Risk Management Files For Medical Device Developers Medical Device Risk Management Plan Covering all elements of the risk management process. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Identify when to use risk management. Rmp aims for the risks of drugs to be evaluated at. Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is. Device Risk Management Plan.
From www.contrapositionmagazine.com
Medical Device Risk Management Report Template Template 2 Resume Device Risk Management Plan Covering all elements of the risk management process. Further, the plan needs to establish how the device risks will be Rmp aims for the risks of drugs to be evaluated at. Discuss the reasons for conducting risk management activities for medical devices. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. This effort led. Device Risk Management Plan.
From www.vrogue.co
Medical Device Risk Management Analysis vrogue.co Device Risk Management Plan Further, the plan needs to establish how the device risks will be Covering all elements of the risk management process. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Identify when. Device Risk Management Plan.
From www.orielstat.com
Preparing a Medical Device Risk Management Review and Report Device Risk Management Plan Identify when to use risk management. Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Further, the plan needs to establish how the device risks will be Risk management plan this is device specific and will be your guiding document for ensuring all of your other. Device Risk Management Plan.
From www.qualio.com
ISO 13485 risk management plan template Device Risk Management Plan Further, the plan needs to establish how the device risks will be Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Identify when to use risk management. Discuss the reasons for. Device Risk Management Plan.
From onethousandvillages.blogspot.com
Iso14971 Risk Management Template Iso 14971 Medical Risk Management Device Risk Management Plan Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Further, the plan needs to establish how the device risks will be Discuss the reasons for conducting risk management activities for medical. Device Risk Management Plan.
From medicaldevicehq.com
Risk Management Plan Template ISO 14971 Device Risk Management Plan Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Covering all elements of the risk management process. Further, the plan needs to establish how the device risks will be Each medical. Device Risk Management Plan.
From www.greenlight.guru
ISO 14971 Risk Management Plan Template [Free Download] Device Risk Management Plan Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Discuss the reasons for conducting risk management activities for medical devices. Further, the plan needs to establish how the device risks will be Risk management for medical devices according to iso 14971 is. Device Risk Management Plan.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Device Risk Management Plan This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Risk management plan this is device specific and will be your guiding document for. Device Risk Management Plan.
From qualcy.com
Medical Device Quality Risk Management · Qualcy eQMS Device Risk Management Plan Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Further, the plan needs to establish how the device risks will be Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk management. Each medical device must have its own risk management plan that identifies the risk management activities. Device Risk Management Plan.
From mungfali.com
Medical Device Risk Management Plan Template Device Risk Management Plan Rmp aims for the risks of drugs to be evaluated at. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Further, the plan. Device Risk Management Plan.
From hydrocodone6canadian6pharmacies6cod.blogspot.com
Iso14971 Risk Management Template / Creating A Medical Device Risk Device Risk Management Plan Identify when to use risk management. Discuss the reasons for conducting risk management activities for medical devices. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Rmp aims for the risks of drugs to be evaluated at. Covering all elements of the. Device Risk Management Plan.
From mungfali.com
Medical Device Risk Management Plan Template Device Risk Management Plan Rmp aims for the risks of drugs to be evaluated at. Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Further, the plan needs to establish how the device risks will. Device Risk Management Plan.
From www.aplyon.com
Risk Management Procedure Device Risk Management Plan Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Identify when to use risk management. Discuss the reasons for conducting risk management activities for medical devices. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur. Device Risk Management Plan.
From qbd.eu
Why Medical Device Risk Management is as complex as it is crucial Device Risk Management Plan Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Further, the plan needs to establish how the device risks will be This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Rmp aims. Device Risk Management Plan.
From www.examples.com
Risk Management Plan 35+ Examples, Format, Pdf Device Risk Management Plan Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Further, the plan needs to establish how the device risks will be This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Covering all elements of the risk management process. Rmp aims for the risks of. Device Risk Management Plan.
From www.vrogue.co
Choosing The Right Medical Device Risk Management Too vrogue.co Device Risk Management Plan Rmp aims for the risks of drugs to be evaluated at. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Risk management for. Device Risk Management Plan.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Device Risk Management Plan Covering all elements of the risk management process. Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Risk management plan this is device specific and will be your guiding document for. Device Risk Management Plan.
From medicaldevicehq.com
How to work with medical device risk management Medical Device HQ Device Risk Management Plan Identify when to use risk management. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Discuss the reasons for conducting risk management activities for medical devices. Covering all elements of the risk management process. Risk management for medical devices according to iso 14971 is essential for. Device Risk Management Plan.
From www.slideteam.net
Risk Management Plan Analysis Report For Electronic Device Device Risk Management Plan Identify when to use risk management. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Rmp aims for the. Device Risk Management Plan.
From www.vrogue.co
Risk Management For Medical Device And Iso 149712019 vrogue.co Device Risk Management Plan This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Covering all elements of the risk management process. Discuss the reasons for conducting risk. Device Risk Management Plan.
From criticalcatalyst.com
Risk Management Under EU Medical Device Regulation CRITICAL CATALYST Device Risk Management Plan Further, the plan needs to establish how the device risks will be Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Identify when to use risk management. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle.. Device Risk Management Plan.
From simbex.com
ISO 14971 Managing Risk in Medical Device Development Simbex Device Risk Management Plan Identify when to use risk management. Covering all elements of the risk management process. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Rmp aims for the risks of drugs to be evaluated at. Discuss the reasons for conducting risk management activities. Device Risk Management Plan.
From www.pinterest.com
Top Hospital Risk Management Plan Template Treatment plan template Device Risk Management Plan Further, the plan needs to establish how the device risks will be This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that. Discuss the reasons for conducting risk. Device Risk Management Plan.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Device Risk Management Plan This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Identify when to use risk management. Further, the plan needs to establish how the device risks will be Risk management provides visually intuitive, collaborative workflows for creating risk matrices and documenting risk and is the only solution in the industry that.. Device Risk Management Plan.
From medicaldevicehq.com
ISO 14971 Risk Management Plan Template Device Risk Management Plan Covering all elements of the risk management process. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Rmp aims for the risks of drugs to be evaluated at. Further, the plan needs to establish how the device risks will be Each medical device must have its. Device Risk Management Plan.
From mavink.com
Ndis Client Risk Assessment Template Device Risk Management Plan Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Rmp aims for the risks of drugs to be evaluated at. This effort led to the first edition of bs en iso. Device Risk Management Plan.
From corsi-dm.it
Introduction to Medical Device Safety Risk Management in Compliance Device Risk Management Plan Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Covering all elements of the risk management process. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. This effort led to the first edition of bs en iso 14971 [1] in. Device Risk Management Plan.
From medicaldevicehq.com
Crash course Project risk management for medical devices Device Risk Management Plan Identify when to use risk management. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each phase of the product lifecycle. Rmp aims for the risks of drugs to be evaluated at. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Risk. Device Risk Management Plan.
From blog.securitymetrics.com
How Much Does a HIPAA Risk Management Plan Cost? Device Risk Management Plan Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Rmp aims for. Device Risk Management Plan.
From trending-right-now49.blogspot.com
Iso14971 Risk Management Template / Aligned Ag Iso 14971 Compliant Risk Device Risk Management Plan Rmp aims for the risks of drugs to be evaluated at. Covering all elements of the risk management process. This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Further, the plan needs to establish how the device risks will be Risk management plan this is device specific and will be. Device Risk Management Plan.
From medicaldevicehq.com
How to integrate proactive safety by design with medical device risk Device Risk Management Plan Further, the plan needs to establish how the device risks will be Discuss the reasons for conducting risk management activities for medical devices. Risk management for medical devices according to iso 14971 is essential for compliance, complementing the. Each medical device must have its own risk management plan that identifies the risk management activities that need to occur at each. Device Risk Management Plan.
From www.linkedin.com
Do you have a Medical Device Risk Management Plan? Device Risk Management Plan Risk management plan this is device specific and will be your guiding document for ensuring all of your other risk activities take place. Further, the plan needs to establish how the device risks will be This effort led to the first edition of bs en iso 14971 [1] in 2000, in which the principles. Discuss the reasons for conducting risk. Device Risk Management Plan.