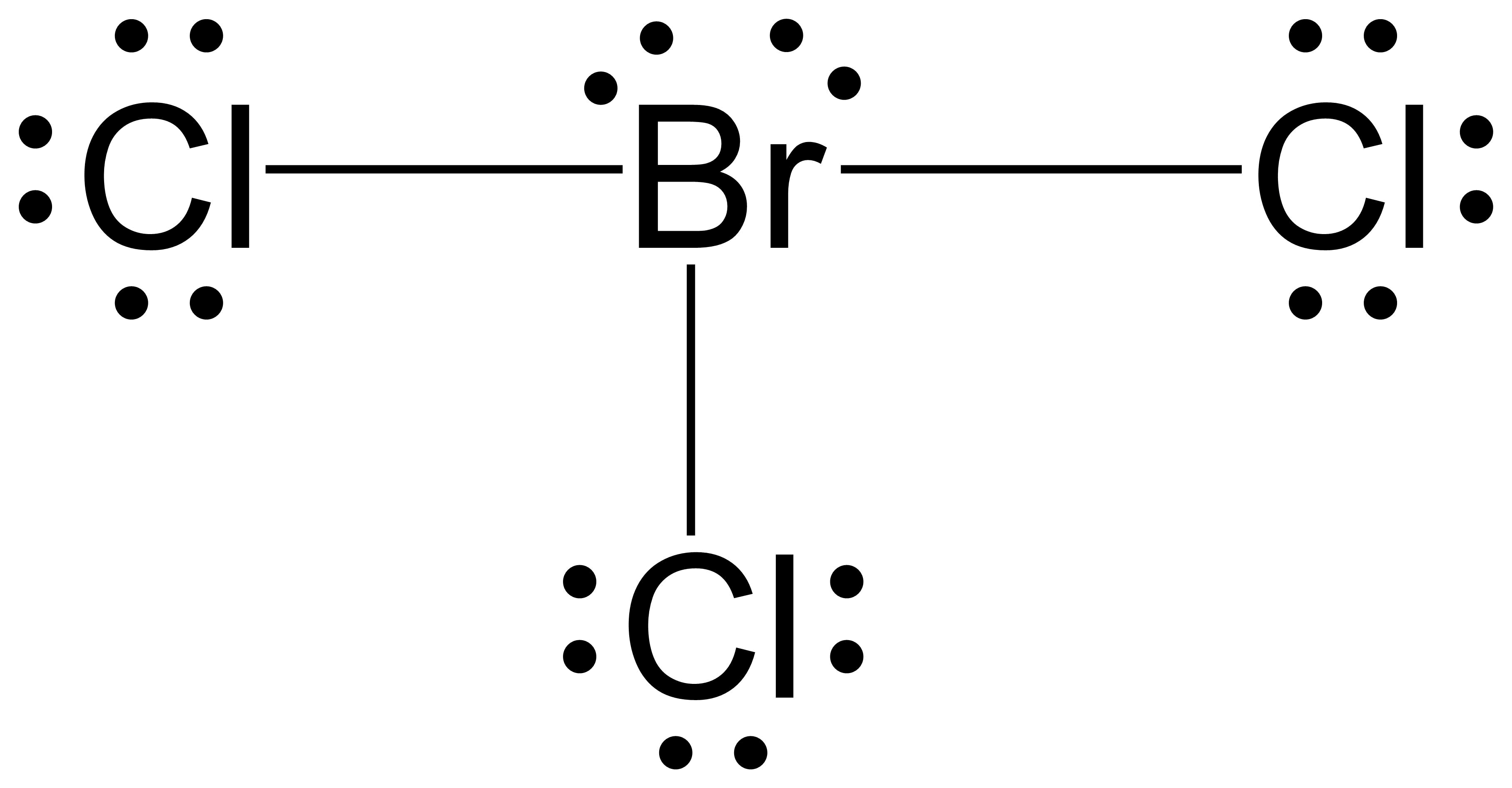Bromine Charge In Ionic Bond . Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. An oxide ion, a −2 charge; These oppositely charged ions attract each other to form ionic networks (or lattices). A sodium ion has a +1 charge; 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. And a bromide ion, a −1 charge. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Lets look at the 4 oxyanions of bromine. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons.
from wisc.pb.unizin.org
An oxide ion, a −2 charge; In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. And a bromide ion, a −1 charge. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. These oppositely charged ions attract each other to form ionic networks (or lattices). Lets look at the 4 oxyanions of bromine. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:
Resonance Structures and Formal Charge (M8Q3) UWMadison Chemistry
Bromine Charge In Ionic Bond These oppositely charged ions attract each other to form ionic networks (or lattices). In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. A sodium ion has a +1 charge; Lets look at the 4 oxyanions of bromine. These oppositely charged ions attract each other to form ionic networks (or lattices). And a bromide ion, a −1 charge. An oxide ion, a −2 charge; For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic.
From slideplayer.com
Ionic and Covalent bonding Chapters 15 and ppt download Bromine Charge In Ionic Bond An oxide ion, a −2 charge; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Lets look at the 4 oxyanions of bromine. In this article, you will learn about the ionic charge, nuclear. Bromine Charge In Ionic Bond.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4982303 Bromine Charge In Ionic Bond In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. These oppositely charged ions attract each other to form ionic networks (or lattices). 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. An oxide ion, a −2 charge;. Bromine Charge In Ionic Bond.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Bromine Charge In Ionic Bond A sodium ion has a +1 charge; An oxide ion, a −2 charge; These oppositely charged ions attract each other to form ionic networks (or lattices). Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Bromine Charge In Ionic Bond.
From kirstenxidawson.blogspot.com
Does Nitrogen and Bromine Form an Ionic Bond Bromine Charge In Ionic Bond A sodium ion has a +1 charge; And a bromide ion, a −1 charge. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. 4.4 the ionic bond model an ionic compound is. Bromine Charge In Ionic Bond.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Bromine Charge In Ionic Bond Lets look at the 4 oxyanions of bromine. For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A sodium ion has a +1 charge; These oppositely charged. Bromine Charge In Ionic Bond.
From quizdbcornwallis.z21.web.core.windows.net
What Is Bromine Charge Bromine Charge In Ionic Bond 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. And a bromide ion, a −1 charge. Bromine naturally forms an anion, a negatively. Bromine Charge In Ionic Bond.
From www.slideserve.com
PPT Ionic Bonds and Ions PowerPoint Presentation, free download ID Bromine Charge In Ionic Bond Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Lets look at the 4 oxyanions of bromine. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. A sodium ion has a +1 charge; Compounds composed of ions are called. Bromine Charge In Ionic Bond.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Bromine Charge In Ionic Bond Lets look at the 4 oxyanions of bromine. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. An oxide ion, a −2 charge; These oppositely charged ions attract each other to form ionic networks (or lattices). A sodium ion has a +1 charge; Bromine naturally forms an. Bromine Charge In Ionic Bond.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Charge In Ionic Bond Lets look at the 4 oxyanions of bromine. These oppositely charged ions attract each other to form ionic networks (or lattices). A sodium ion has a +1 charge; For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. Compounds composed of ions are called ionic compounds (or salts),. Bromine Charge In Ionic Bond.
From slidetodoc.com
PART I Bonding Basics Bonding is an example Bromine Charge In Ionic Bond These oppositely charged ions attract each other to form ionic networks (or lattices). A sodium ion has a +1 charge; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Lets look at the 4 oxyanions of bromine. Bromine naturally forms an anion, a negatively charged ion, when it gets. Bromine Charge In Ionic Bond.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Bromine Charge In Ionic Bond For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. These oppositely charged ions attract each other to form ionic networks (or lattices). A sodium ion has a +1 charge; Lets look at the 4 oxyanions of bromine. 4.4 the ionic bond model an ionic compound is made. Bromine Charge In Ionic Bond.
From www.slideshare.net
Ionic Bonds Chapter 7 Bromine Charge In Ionic Bond Lets look at the 4 oxyanions of bromine. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. For example, the neutral bromine atom,. Bromine Charge In Ionic Bond.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Bromine Charge In Ionic Bond For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. Lets look at the 4 oxyanions of bromine. And a bromide ion, a −1 charge. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Bromine. Bromine Charge In Ionic Bond.
From brainly.com
Which of the following elements is most likely to form an ionic bond Bromine Charge In Ionic Bond Lets look at the 4 oxyanions of bromine. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. These oppositely charged ions attract each other to form ionic networks (or lattices). In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. A sodium ion has a +1 charge;. Bromine Charge In Ionic Bond.
From slideplayer.com
Chemical Bonds. ppt download Bromine Charge In Ionic Bond A sodium ion has a +1 charge; Lets look at the 4 oxyanions of bromine. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. For the interaction of. Bromine Charge In Ionic Bond.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Bromine Charge In Ionic Bond An oxide ion, a −2 charge; In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for.. Bromine Charge In Ionic Bond.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Bromine Charge In Ionic Bond Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. And a bromide ion, a −1 charge. A sodium ion has a +1 charge; Lets look at the 4 oxyanions of. Bromine Charge In Ionic Bond.
From www.coursehero.com
[Solved] The formal charge on the bromine atom in BrO 3 drawn with Bromine Charge In Ionic Bond Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Lets look at the 4 oxyanions of bromine. And a bromide ion, a −1 charge. For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. An oxide ion, a −2 charge; These oppositely. Bromine Charge In Ionic Bond.
From app.emaze.com
Bromine ) on emaze Bromine Charge In Ionic Bond In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. These oppositely charged ions attract each other to form ionic networks (or lattices). And a bromide ion, a −1 charge. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. A. Bromine Charge In Ionic Bond.
From www.numerade.com
SOLVED Question 30 A bromine molecule (Bri2 has Ipolar covalent bond Bromine Charge In Ionic Bond 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. An oxide ion, a −2 charge; Compounds composed of ions are called ionic compounds (or salts), and their constituent. Bromine Charge In Ionic Bond.
From dokumen.tips
(PDF) WS, Ionic Bonding Edl Bonding WS Element Gain or Lose of Bromine Charge In Ionic Bond In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. And a bromide ion, a −1 charge. These oppositely charged ions attract each other to form ionic networks (or lattices). Lets look at the 4 oxyanions of bromine. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are. Bromine Charge In Ionic Bond.
From wisc.pb.unizin.org
Resonance Structures and Formal Charge (M8Q3) UWMadison Chemistry Bromine Charge In Ionic Bond Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by. Bromine Charge In Ionic Bond.
From h-o-m-e.org
Ionic Bond Understanding the Force of Attraction Bromine Charge In Ionic Bond For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Lets look at the 4 oxyanions of bromine. A sodium ion has a +1 charge; An oxide ion, a −2 charge; Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. These oppositely. Bromine Charge In Ionic Bond.
From h-o-m-e.org
Bromide (Br) Ion Charges Explained Bromine Charge In Ionic Bond 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical. Bromine Charge In Ionic Bond.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Bromine Charge In Ionic Bond And a bromide ion, a −1 charge. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. For the interaction of a sodium ion with an oxide ion, q 1 =. Bromine Charge In Ionic Bond.
From www.slideserve.com
PPT Chemistry Unit 2 PowerPoint Presentation, free download ID3668529 Bromine Charge In Ionic Bond An oxide ion, a −2 charge; These oppositely charged ions attract each other to form ionic networks (or lattices). Lets look at the 4 oxyanions of bromine. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Compounds composed of ions are called ionic compounds (or salts), and. Bromine Charge In Ionic Bond.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Bromine Charge In Ionic Bond Lets look at the 4 oxyanions of bromine. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. And a bromide ion, a −1 charge. For example, the. Bromine Charge In Ionic Bond.
From www.showme.com
Aluminum and Bromine Bond Science ShowMe Bromine Charge In Ionic Bond These oppositely charged ions attract each other to form ionic networks (or lattices). Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. An oxide ion, a −2 charge; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A sodium ion has a +1. Bromine Charge In Ionic Bond.
From h-o-m-e.org
Decoding The Ionic Charge of Bromine Bromine Charge In Ionic Bond A sodium ion has a +1 charge; 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: And a bromide ion, a −1 charge. An oxide. Bromine Charge In Ionic Bond.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Charge In Ionic Bond Lets look at the 4 oxyanions of bromine. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. Bromine naturally forms an anion, a negatively charged ion, when it. Bromine Charge In Ionic Bond.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Charge In Ionic Bond 4.4 the ionic bond model an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. Lets look at the 4 oxyanions of bromine. For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. A sodium ion has a +1. Bromine Charge In Ionic Bond.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Charge In Ionic Bond In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. And a bromide ion, a −1 charge. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. Bromine Charge In Ionic Bond.
From derekcarrsavvy-chemist.blogspot.co.uk
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Bromine Charge In Ionic Bond In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: For the interaction of a sodium ion with an oxide ion, q 1 = +1 and q 2 = −2, whereas for. An. Bromine Charge In Ionic Bond.
From www.showme.com
Ionic Bonding calcium and bromine Science ShowMe Bromine Charge In Ionic Bond In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. And a bromide ion, a −1 charge. A sodium ion has a +1 charge; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: For the interaction of a sodium ion with an. Bromine Charge In Ionic Bond.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Charge In Ionic Bond An oxide ion, a −2 charge; Lets look at the 4 oxyanions of bromine. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. These oppositely charged ions attract each other to form ionic networks (or lattices). For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron. Bromine Charge In Ionic Bond.