How Will A Solution Change If A Base Is Added . the basic buffer is prepared by mixing a weak base and its salt with a strong acid. If the dissociation constant of the acid (pk. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Buffer action, in general, is defined as the ability of the buffer solution to resist. A buffer solution consists of a.
from saylordotorg.github.io
A buffer solution consists of a. the basic buffer is prepared by mixing a weak base and its salt with a strong acid. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. If the dissociation constant of the acid (pk. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. Buffer action, in general, is defined as the ability of the buffer solution to resist. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)).
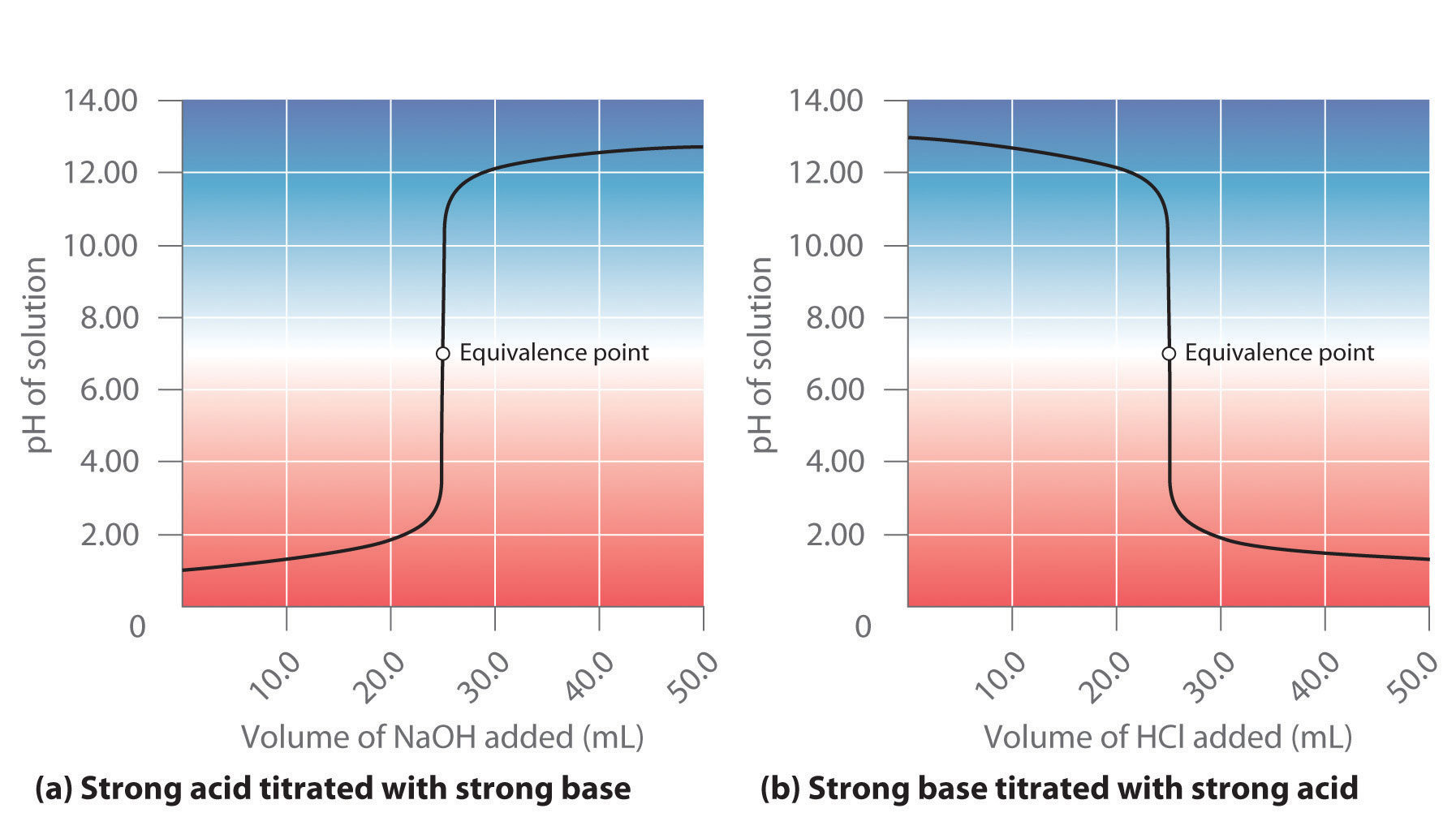
AcidBase Titrations
How Will A Solution Change If A Base Is Added If the dissociation constant of the acid (pk. an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. the basic buffer is prepared by mixing a weak base and its salt with a strong acid. If the dissociation constant of the acid (pk. Buffer action, in general, is defined as the ability of the buffer solution to resist. A buffer solution consists of a.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps How Will A Solution Change If A Base Is Added a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. buffer solutions resist a change in ph when small amounts. How Will A Solution Change If A Base Is Added.
From printablekinonasw.z14.web.core.windows.net
How To Calculate Ph In Chemistry How Will A Solution Change If A Base Is Added the basic buffer is prepared by mixing a weak base and its salt with a strong acid. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. How Will A Solution Change If A Base Is Added.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation ID2499044 How Will A Solution Change If A Base Is Added if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. Buffer action, in general, is defined as the ability of the buffer solution to resist. A buffer solution consists of a. an aqueous solution that resists any change in ph by. How Will A Solution Change If A Base Is Added.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW How Will A Solution Change If A Base Is Added a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer solution consists of a. the basic buffer is prepared by mixing a weak base and its salt with a strong acid. buffer solutions resist a change in ph when small amounts. How Will A Solution Change If A Base Is Added.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content How Will A Solution Change If A Base Is Added an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). If the dissociation constant of the acid (pk. A buffer solution consists of. How Will A Solution Change If A Base Is Added.
From www.slideserve.com
PPT A solution is a homogeneous mixture of two or more substances How Will A Solution Change If A Base Is Added Buffer action, in general, is defined as the ability of the buffer solution to resist. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base. How Will A Solution Change If A Base Is Added.
From srknjpmlgqnkz.blogspot.com
Change Of Base Formula Examples, Change Of Base Formula Or Rule How Will A Solution Change If A Base Is Added the basic buffer is prepared by mixing a weak base and its salt with a strong acid. an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. A buffer solution consists of a. If the dissociation constant of the acid (pk. Buffer action, in general,. How Will A Solution Change If A Base Is Added.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Will A Solution Change If A Base Is Added a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. Buffer action, in general, is defined as the ability of the. How Will A Solution Change If A Base Is Added.
From www.nagwa.com
Question Video Identifying the Graph Showing Change in pH When Mixing How Will A Solution Change If A Base Is Added if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). the basic buffer is prepared by mixing a. How Will A Solution Change If A Base Is Added.
From www.sliderbase.com
Titration How Will A Solution Change If A Base Is Added the basic buffer is prepared by mixing a weak base and its salt with a strong acid. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. If the dissociation constant of the acid (pk. Buffer action, in general, is defined. How Will A Solution Change If A Base Is Added.
From www.ocmsolution.com
OCM Solution Change Management Project Starter Kit OCM Solution How Will A Solution Change If A Base Is Added Buffer action, in general, is defined as the ability of the buffer solution to resist. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. If the dissociation constant of the acid (pk. buffer solutions resist a change in ph when. How Will A Solution Change If A Base Is Added.
From saylordotorg.github.io
AcidBase Titrations How Will A Solution Change If A Base Is Added an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. If the dissociation constant of the acid (pk. Buffer action, in general, is defined as the ability of the buffer solution to resist. A buffer solution consists of a. buffer solutions resist a change in. How Will A Solution Change If A Base Is Added.
From fr.wikihow.com
5 manières de calculer la concentration d'une solution How Will A Solution Change If A Base Is Added buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. If the dissociation constant of the acid (pk. Buffer action, in general, is defined as the ability of the buffer solution to resist. if we add a base (hydroxide ions), ammonium ions in the buffer react with the. How Will A Solution Change If A Base Is Added.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium How Will A Solution Change If A Base Is Added buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Buffer action, in general, is defined as the ability of the buffer solution to. How Will A Solution Change If A Base Is Added.
From chemistryguru.com.sg
Determine pH of Resultant Solution of AcidBase Reaction How Will A Solution Change If A Base Is Added an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. A buffer solution consists of a. if we add a base (hydroxide ions), ammonium. How Will A Solution Change If A Base Is Added.
From www.numerade.com
SOLVED Question 20 The function of a buffer is to maintain the pH of a How Will A Solution Change If A Base Is Added A buffer solution consists of a. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Buffer action, in general, is defined as the ability of the buffer solution to resist. buffer solutions resist a change in ph when small amounts of a strong. How Will A Solution Change If A Base Is Added.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube How Will A Solution Change If A Base Is Added Buffer action, in general, is defined as the ability of the buffer solution to resist. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. If the dissociation constant of the acid (pk. buffer solutions resist a change in ph when small amounts of a strong acid or. How Will A Solution Change If A Base Is Added.
From www.vrogue.co
Solution Acid Base Titration Method Studypool vrogue.co How Will A Solution Change If A Base Is Added the basic buffer is prepared by mixing a weak base and its salt with a strong acid. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. buffer solutions resist a change in ph when small amounts of a strong acid or a. How Will A Solution Change If A Base Is Added.
From www.echemi.com
What would be the pH of 0.01M solution of NH4CL in water at 25 degree How Will A Solution Change If A Base Is Added an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. A buffer solution consists of a. the basic buffer is prepared by mixing a weak base and its salt with a strong acid. If the dissociation constant of the acid (pk. a solution whose. How Will A Solution Change If A Base Is Added.
From saylordotorg.github.io
AcidBase Titrations How Will A Solution Change If A Base Is Added if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. the basic buffer is prepared by mixing a weak base and its salt with a strong acid. a solution whose ph is not altered to any great extent by the. How Will A Solution Change If A Base Is Added.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG How Will A Solution Change If A Base Is Added buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). Buffer action, in general, is defined as the ability of the buffer solution to resist. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. A. How Will A Solution Change If A Base Is Added.
From www.vedantu.com
In an acidbase titration, 0.1M HCl solution was added to the NaOH How Will A Solution Change If A Base Is Added If the dissociation constant of the acid (pk. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. A buffer solution consists of a. buffer solutions resist a change in ph when small amounts of a strong acid or a strong. How Will A Solution Change If A Base Is Added.
From www.slideshare.net
2012 topic 18 2 buffer solutions How Will A Solution Change If A Base Is Added If the dissociation constant of the acid (pk. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. if we add a. How Will A Solution Change If A Base Is Added.
From www.youtube.com
pH of Dilute Solution (Example) YouTube How Will A Solution Change If A Base Is Added If the dissociation constant of the acid (pk. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). the basic buffer. How Will A Solution Change If A Base Is Added.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein How Will A Solution Change If A Base Is Added buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). the basic buffer is prepared by mixing a weak base and its salt with a strong acid. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to. How Will A Solution Change If A Base Is Added.
From www.teachoo.com
[MCQ] The graph given below depicts a neutralization reaction (acid How Will A Solution Change If A Base Is Added an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. the basic buffer is prepared by mixing a weak base. How Will A Solution Change If A Base Is Added.
From www.nagwa.com
Question Video Identifying the Color of a Solution Containing the Acid How Will A Solution Change If A Base Is Added buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A buffer solution consists of a. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a solution whose ph is not altered to any. How Will A Solution Change If A Base Is Added.
From www.nagwa.com
Question Video Identifying the Color of the Product Solution When a How Will A Solution Change If A Base Is Added buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. If the dissociation constant of the acid (pk. Buffer action, in general, is defined as the. How Will A Solution Change If A Base Is Added.
From byjus.com
You are provided with three test tubes A, B and C as shown in Figure How Will A Solution Change If A Base Is Added Buffer action, in general, is defined as the ability of the buffer solution to resist. A buffer solution consists of a. the basic buffer is prepared by mixing a weak base and its salt with a strong acid. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added.. How Will A Solution Change If A Base Is Added.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts How Will A Solution Change If A Base Is Added Buffer action, in general, is defined as the ability of the buffer solution to resist. A buffer solution consists of a. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). buffer solutions resist a change in ph when small amounts of a strong acid or. How Will A Solution Change If A Base Is Added.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps How Will A Solution Change If A Base Is Added buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. Buffer action, in general, is defined as the ability of the buffer solution to resist. . How Will A Solution Change If A Base Is Added.
From chem.libretexts.org
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts How Will A Solution Change If A Base Is Added If the dissociation constant of the acid (pk. an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Buffer action, in. How Will A Solution Change If A Base Is Added.
From chemistry.com.pk
Colours of Transition Metal Ions in Aqueous Solution [Infographic How Will A Solution Change If A Base Is Added the basic buffer is prepared by mixing a weak base and its salt with a strong acid. an aqueous solution that resists any change in ph by adding a small amount of acid or base is called a buffer solution. Buffer action, in general, is defined as the ability of the buffer solution to resist. if we. How Will A Solution Change If A Base Is Added.
From opentextbc.ca
14.6 Buffers Chemistry How Will A Solution Change If A Base Is Added If the dissociation constant of the acid (pk. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base. How Will A Solution Change If A Base Is Added.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Will A Solution Change If A Base Is Added if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a solution whose ph is not altered to any great. How Will A Solution Change If A Base Is Added.