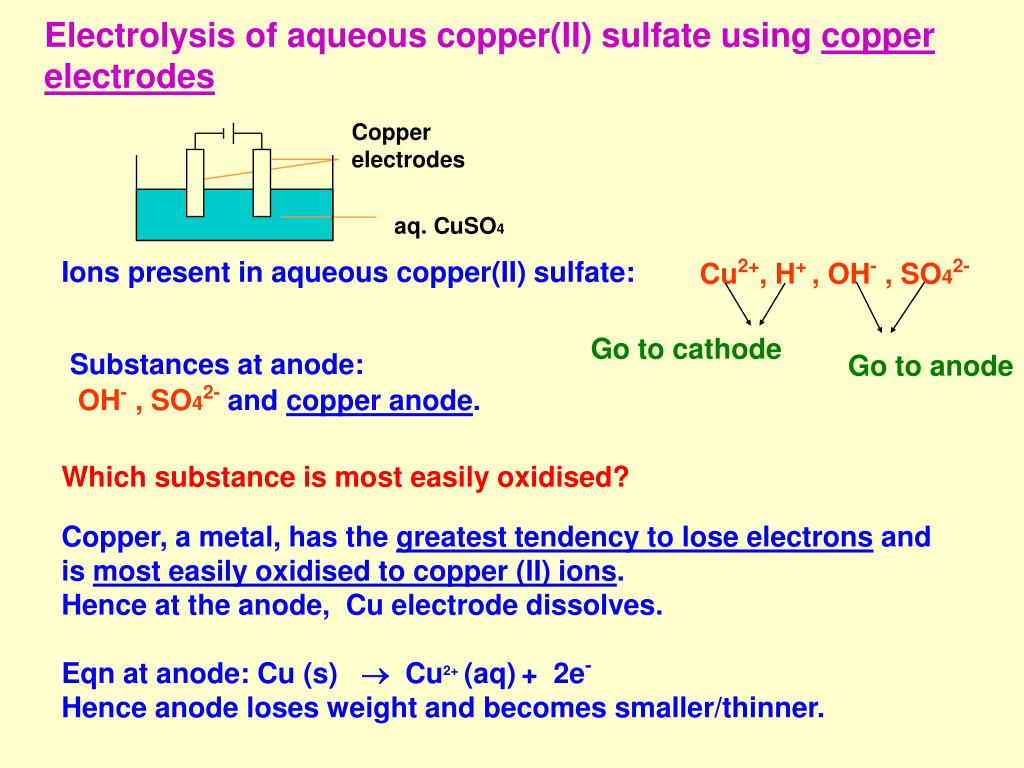
Reactive Electrodes Examples . In the early years after development of sacrificial electrode. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. examples and synthetic application. Electroplating is the process of applying one metal onto another using an. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. Examples of reactive electrodes are copper, silver and. reactive electrodes are mainly used in electroplating. graphite and platinum are examples of inert electrodes.
from www.slideserve.com
In the early years after development of sacrificial electrode. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. reactive electrodes are mainly used in electroplating. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. Examples of reactive electrodes are copper, silver and. graphite and platinum are examples of inert electrodes. examples and synthetic application. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Electroplating is the process of applying one metal onto another using an. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the.
PPT Electrolysis PowerPoint Presentation, free download ID5813936
Reactive Electrodes Examples in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. In the early years after development of sacrificial electrode. graphite and platinum are examples of inert electrodes. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. examples and synthetic application. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. reactive electrodes are mainly used in electroplating. Electroplating is the process of applying one metal onto another using an. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. Examples of reactive electrodes are copper, silver and.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Reactive Electrodes Examples reactive electrodes are mainly used in electroplating. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. In the early years after development of sacrificial electrode. revision. Reactive Electrodes Examples.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Reactive Electrodes Examples examples and synthetic application. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Examples of reactive electrodes are copper, silver and. In the early years after development of sacrificial electrode. reactive electrodes are those electrodes which take part in the reaction taking place. Reactive Electrodes Examples.
From www.slideserve.com
PPT UNIT V PowerPoint Presentation, free download ID4322725 Reactive Electrodes Examples Electroplating is the process of applying one metal onto another using an. graphite and platinum are examples of inert electrodes. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. reactive electrodes are mainly used in electroplating. revision notes on electrolysis of aqueous. Reactive Electrodes Examples.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Reactive Electrodes Examples graphite and platinum are examples of inert electrodes. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. reactive electrodes are mainly used in electroplating. Electroplating is the process of. Reactive Electrodes Examples.
From www.youtube.com
Electrolysis of Copper Reactive Electrode 5070/0620 YouTube Reactive Electrodes Examples Electroplating is the process of applying one metal onto another using an. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Examples of reactive electrodes are copper, silver and. In the early years after development of sacrificial electrode. graphite and platinum are examples of. Reactive Electrodes Examples.
From www.youtube.com
Upper Sec IP Chem Electrolysis Electrolysis Using Reactive Reactive Electrodes Examples in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. graphite and platinum are examples of inert electrodes. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. In the early years after development of sacrificial electrode. . Reactive Electrodes Examples.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Reactive Electrodes Examples in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive electrodes are mainly used in electroplating. Electroplating is the process of applying one metal onto another using an. graphite and platinum are examples of inert electrodes. In the early years after development of sacrificial electrode. examples and synthetic application.. Reactive Electrodes Examples.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 Reactive Electrodes Examples In the early years after development of sacrificial electrode. Examples of reactive electrodes are copper, silver and. reactive electrodes are mainly used in electroplating. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. revision notes on electrolysis of aqueous solutions for the cambridge. Reactive Electrodes Examples.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Reactive Electrodes Examples examples and synthetic application. graphite and platinum are examples of inert electrodes. Electroplating is the process of applying one metal onto another using an. reactive electrodes are mainly used in electroplating. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. revision notes on electrolysis of aqueous solutions for. Reactive Electrodes Examples.
From byjus.com
What is an active electrode? Explain with the help of an example Reactive Electrodes Examples In the early years after development of sacrificial electrode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. for example, when molten kcl is subjected to electrolysis. Reactive Electrodes Examples.
From ppt-online.org
Electrolysis презентация онлайн Reactive Electrodes Examples Electroplating is the process of applying one metal onto another using an. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. graphite and platinum are examples of inert electrodes. In the early years after development of sacrificial electrode. examples and synthetic application. reactive electrodes are those electrodes which take. Reactive Electrodes Examples.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Reactive Electrodes Examples reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. Electroplating is the process of applying one metal onto another using an. Examples of reactive. Reactive Electrodes Examples.
From www.chegg.com
Solved 0/1 point Tap on the reactive electrode flow of Reactive Electrodes Examples reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Electroplating is the process of applying one metal onto another using an. in any. Reactive Electrodes Examples.
From www.youtube.com
AD Online Electrolysis Concept 7 Electrolysis Using Inert VS Reactive Electrodes Examples In the early years after development of sacrificial electrode. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. Examples of reactive electrodes are copper, silver and. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get. Reactive Electrodes Examples.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Reactive Electrodes Examples Examples of reactive electrodes are copper, silver and. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. reactive electrodes are mainly used in electroplating. Electroplating is the process of applying one metal onto another using an. In the early years after development of sacrificial. Reactive Electrodes Examples.
From www.researchgate.net
Microglial reaction surrounding electrode ramified and reactive Reactive Electrodes Examples examples and synthetic application. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. Electroplating is the process of applying one metal onto another using an. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save. Reactive Electrodes Examples.
From www.youtube.com
Electrochemistry 6 Type of Electrodes YouTube Reactive Electrodes Examples In the early years after development of sacrificial electrode. graphite and platinum are examples of inert electrodes. examples and synthetic application. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written. Reactive Electrodes Examples.
From www.chemistry-teaching-resources.com
chemistry picture Reactive Electrodes Examples Examples of reactive electrodes are copper, silver and. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. In the early years after development of sacrificial electrode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive. Reactive Electrodes Examples.
From www.simplechemconcepts.com
Simple Cells in Electrolysis topic Reactive Electrodes Examples examples and synthetic application. graphite and platinum are examples of inert electrodes. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. Electroplating is the process of applying one metal onto another using an. Examples of reactive electrodes are copper, silver and. revision notes on electrolysis. Reactive Electrodes Examples.
From www.slideserve.com
PPT UNIT V PowerPoint Presentation, free download ID4322725 Reactive Electrodes Examples Examples of reactive electrodes are copper, silver and. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. in any electrochemical cell (electrolytic or. Reactive Electrodes Examples.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Reactive Electrodes Examples reactive electrodes are mainly used in electroplating. examples and synthetic application. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. graphite and platinum are examples. Reactive Electrodes Examples.
From www.slideserve.com
PPT UNIT V PowerPoint Presentation, free download ID4322725 Reactive Electrodes Examples examples and synthetic application. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions. Reactive Electrodes Examples.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Reactive Electrodes Examples reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. graphite and platinum are examples of inert electrodes. Examples of reactive electrodes are copper, silver and. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save. Reactive Electrodes Examples.
From dxotaboqx.blob.core.windows.net
Medical Electrodes Types at Marian Mask blog Reactive Electrodes Examples graphite and platinum are examples of inert electrodes. reactive electrodes are mainly used in electroplating. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can. Reactive Electrodes Examples.
From mmerevise.co.uk
Electrolysis Worksheets and Revision MME Reactive Electrodes Examples graphite and platinum are examples of inert electrodes. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. reactive electrodes are mainly used in electroplating. Electroplating is. Reactive Electrodes Examples.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Reactive Electrodes Examples Electroplating is the process of applying one metal onto another using an. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. Examples of reactive electrodes are copper, silver and. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. for. Reactive Electrodes Examples.
From www.youtube.com
Electrolysis (reactive electrode) O level chemistry by Ms Chew YouTube Reactive Electrodes Examples reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is. Reactive Electrodes Examples.
From courses.lumenlearning.com
Galvanic Cells Chemistry Reactive Electrodes Examples revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. Examples of reactive electrodes are copper, silver and. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. for example, when molten kcl is. Reactive Electrodes Examples.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 Reactive Electrodes Examples Examples of reactive electrodes are copper, silver and. examples and synthetic application. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. Electroplating is the process of applying one metal onto another using an. for example, when molten kcl is subjected to electrolysis using inert electrodes, the. Reactive Electrodes Examples.
From www.slideserve.com
PPT UNIT V PowerPoint Presentation, free download ID4322725 Reactive Electrodes Examples Electroplating is the process of applying one metal onto another using an. graphite and platinum are examples of inert electrodes. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the. Reactive Electrodes Examples.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Reactive Electrodes Examples Electroplating is the process of applying one metal onto another using an. graphite and platinum are examples of inert electrodes. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. In the early years after development of sacrificial electrode. revision notes on electrolysis of aqueous solutions for the cambridge o level. Reactive Electrodes Examples.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Reactive Electrodes Examples for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. In the early years after development of sacrificial electrode. revision notes on electrolysis of aqueous solutions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. examples and. Reactive Electrodes Examples.
From www.goodscience.com.au
Extraction of Metals Good Science Reactive Electrodes Examples In the early years after development of sacrificial electrode. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. Examples of reactive electrodes are copper, silver and. examples and synthetic application. Electroplating is the process of applying one metal onto another using an. graphite. Reactive Electrodes Examples.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Reactive Electrodes Examples Examples of reactive electrodes are copper, silver and. examples and synthetic application. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. Electroplating is the process of applying one metal onto another using an. graphite and platinum are examples of inert electrodes. revision. Reactive Electrodes Examples.
From courses.lumenlearning.com
17.2 Galvanic Cells (Voltaic Cells) General College Chemistry II Reactive Electrodes Examples graphite and platinum are examples of inert electrodes. for example, when molten kcl is subjected to electrolysis using inert electrodes, the k + ions migrate towards cathode and get reduced to. reactive electrodes are those electrodes which take part in the reaction taking place in the cell and can dissolve in. revision notes on electrolysis of. Reactive Electrodes Examples.