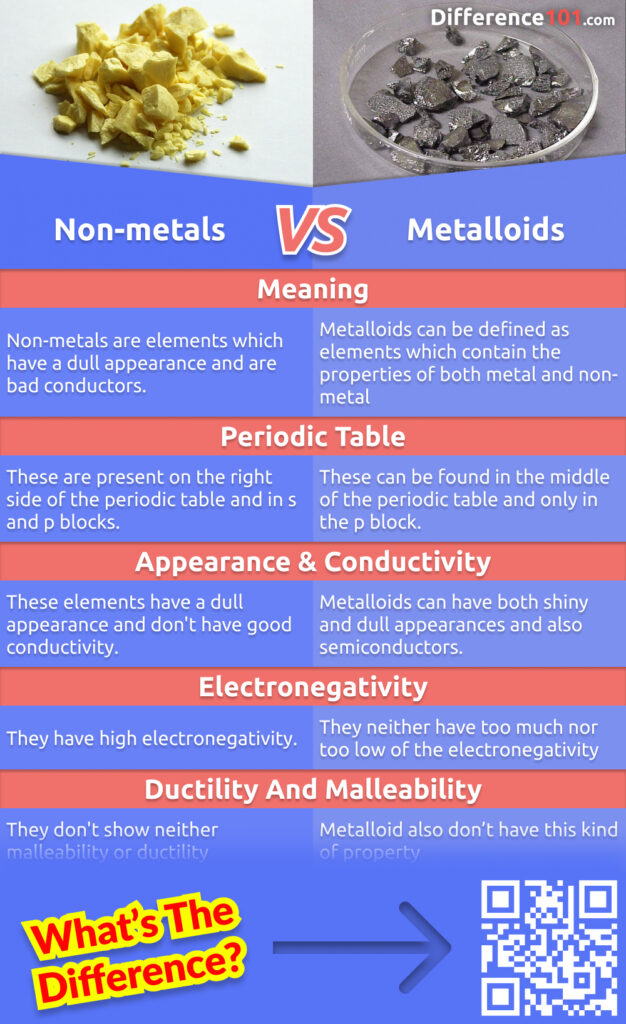
Are Metalloids Lustrous Or Dull . Physically, they are shiny, brittle solids with intermediate to relatively good electrical. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. Arsenic, a metalloid, exhibits a metallic luster, unlike. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. metalloids usually look like metals but behave largely like nonmetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle.
from www.difference101.com
— metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. metalloids usually look like metals but behave largely like nonmetals. Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi.
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons
Are Metalloids Lustrous Or Dull metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metalloids usually look like metals but behave largely like nonmetals. Arsenic, a metalloid, exhibits a metallic luster, unlike.
From slideplayer.com
Atomic Models. ppt download Are Metalloids Lustrous Or Dull Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metalloids usually look like metals but behave largely like nonmetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the. Are Metalloids Lustrous Or Dull.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Are Metalloids Lustrous Or Dull Arsenic, a metalloid, exhibits a metallic luster, unlike. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they. Are Metalloids Lustrous Or Dull.
From www.museoinclusivo.com
Is Aluminum a Metalloid? Exploring the Benefits and Disadvantages of Are Metalloids Lustrous Or Dull some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metalloids. Are Metalloids Lustrous Or Dull.
From slideplayer.com
Unit 1 Chemistry and Matter Mrs. Taylor HASD ppt download Are Metalloids Lustrous Or Dull — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. metalloids usually look like metals but behave largely like nonmetals. . Are Metalloids Lustrous Or Dull.
From www.teachoo.com
Physical Properties of Metals and Non Metals Full list Teachoo Are Metalloids Lustrous Or Dull metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle solids with. Are Metalloids Lustrous Or Dull.
From slideplayer.com
by Steven S. Zumdahl & Donald J. DeCoste University of Illinois ppt Are Metalloids Lustrous Or Dull — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metalloids usually look like metals but behave largely like nonmetals. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. . Are Metalloids Lustrous Or Dull.
From slideplayer.com
What has been presented? ppt video online download Are Metalloids Lustrous Or Dull metalloids usually look like metals but behave largely like nonmetals. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Arsenic, a metalloid, exhibits a metallic luster, unlike. metals tend to. Are Metalloids Lustrous Or Dull.
From www.youtube.com
Metalloids and their Properties Metalloids Periodic Table Are Metalloids Lustrous Or Dull Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Arsenic, a metalloid, exhibits a. Are Metalloids Lustrous Or Dull.
From slideplayer.com
The Periodic Table of Elements ppt download Are Metalloids Lustrous Or Dull Physically, they are shiny, brittle solids with intermediate to relatively good electrical. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. metalloids usually look like metals but behave largely like nonmetals. Arsenic, a metalloid, exhibits a metallic luster, unlike. Some metalloids, such as silicon. Are Metalloids Lustrous Or Dull.
From slideplayer.com
Periodic table Groups and periods ppt download Are Metalloids Lustrous Or Dull Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. Arsenic, a metalloid, exhibits a metallic luster, unlike. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they. Are Metalloids Lustrous Or Dull.
From www.thoughtco.com
Metals, Nonmetals, and Metalloids Worksheet Are Metalloids Lustrous Or Dull Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. metalloids usually look like. Are Metalloids Lustrous Or Dull.
From slideplayer.com
Elements, Atoms & Ions Chapter 4 ppt download Are Metalloids Lustrous Or Dull Physically, they are shiny, brittle solids with intermediate to relatively good electrical. metalloids usually look like metals but behave largely like nonmetals. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they. Are Metalloids Lustrous Or Dull.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Lustrous Or Dull metalloids usually look like metals but behave largely like nonmetals. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. — metalloids can. Are Metalloids Lustrous Or Dull.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Are Metalloids Lustrous Or Dull Physically, they are shiny, brittle solids with intermediate to relatively good electrical. metalloids usually look like metals but behave largely like nonmetals. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Arsenic, a metalloid, exhibits a metallic luster, unlike. metals tend to be lustrous and. Are Metalloids Lustrous Or Dull.
From myroomismylife.blogspot.com
Metals Periodic Table Properties Are Metalloids Lustrous Or Dull metalloids usually look like metals but behave largely like nonmetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. Arsenic, a metalloid, exhibits a metallic luster, unlike. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull. Are Metalloids Lustrous Or Dull.
From www.slideserve.com
PPT The Greeks History of the Atom PowerPoint Presentation, free Are Metalloids Lustrous Or Dull Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. — metalloids. Are Metalloids Lustrous Or Dull.
From www.thoughtco.com
The Difference Between Metals and Nonmetals Are Metalloids Lustrous Or Dull Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle solids with. Are Metalloids Lustrous Or Dull.
From www.chegg.com
Solved Elements found to the right of the metalloids on the Are Metalloids Lustrous Or Dull Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. metalloids usually look like metals but behave largely like nonmetals. Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. some metalloids, such as silicon and germanium,. Are Metalloids Lustrous Or Dull.
From dxowxlvlf.blob.core.windows.net
Recall Where Metalloids Are Found On The Periodic Table at Gary Petree blog Are Metalloids Lustrous Or Dull some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. — metalloids can appear. Are Metalloids Lustrous Or Dull.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Metalloids Lustrous Or Dull Physically, they are shiny, brittle solids with intermediate to relatively good electrical. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Arsenic, a metalloid, exhibits a metallic luster, unlike. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they. Are Metalloids Lustrous Or Dull.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Lustrous Or Dull — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metalloids usually look like metals but behave largely like nonmetals. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Arsenic, a metalloid, exhibits a metallic luster, unlike. Some metalloids, such as. Are Metalloids Lustrous Or Dull.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Are Metalloids Lustrous Or Dull some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases. Are Metalloids Lustrous Or Dull.
From slideplayer.com
Unit 4 Chapter Periodic Table Part ppt download Are Metalloids Lustrous Or Dull — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. metalloids usually look like metals but behave largely like nonmetals. Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle. Are Metalloids Lustrous Or Dull.
From opengeology.org
Earth Materials Mineral Identification Historical Geology Are Metalloids Lustrous Or Dull metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called. Are Metalloids Lustrous Or Dull.
From slideplayer.com
How the periodic table is put together ppt download Are Metalloids Lustrous Or Dull — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. Some metalloids, such. Are Metalloids Lustrous Or Dull.
From slideplayer.com
The Periodic Table. ppt download Are Metalloids Lustrous Or Dull metalloids usually look like metals but behave largely like nonmetals. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Some metalloids, such as silicon and germanium, can act as. Are Metalloids Lustrous Or Dull.
From slideplayer.com
Warm Up, December 1, ) How many protons, neutrons, and electrons are in Are Metalloids Lustrous Or Dull Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. metals. Are Metalloids Lustrous Or Dull.
From slideplayer.com
UNIT 5 Patterns and Compounds ppt download Are Metalloids Lustrous Or Dull Arsenic, a metalloid, exhibits a metallic luster, unlike. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. metalloids usually look like metals but behave largely like nonmetals. some metalloids, such as. Are Metalloids Lustrous Or Dull.
From www.youtube.com
Difference between Metals , Metalloids , and Nonmetals YouTube Are Metalloids Lustrous Or Dull some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metalloids usually look like metals but behave largely like nonmetals. metals tend to be lustrous and shiny, but nonmetals tend to. Are Metalloids Lustrous Or Dull.
From slideplayer.com
Islamic University Gaza ppt download Are Metalloids Lustrous Or Dull — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle.. Are Metalloids Lustrous Or Dull.
From slideshare.net
Metals, nonmetals, metalloids Are Metalloids Lustrous Or Dull — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. Arsenic, a metalloid, exhibits a metallic luster, unlike. metalloids usually look like metals but behave largely like nonmetals. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Some metalloids, such as. Are Metalloids Lustrous Or Dull.
From www.thoughtco.com
Metals, Nonmetals, and Metalloids Worksheet Are Metalloids Lustrous Or Dull Arsenic, a metalloid, exhibits a metallic luster, unlike. some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. metalloids usually look like metals. Are Metalloids Lustrous Or Dull.
From lessonfullpurposive.z22.web.core.windows.net
List Of Properties Of Metals Are Metalloids Lustrous Or Dull Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. metalloids usually look like metals but behave largely like nonmetals. Physically, they are shiny, brittle solids with intermediate to relatively good electrical. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. . Are Metalloids Lustrous Or Dull.
From slideplayer.com
Bellringer Use your periodic table to identify the following Are Metalloids Lustrous Or Dull Physically, they are shiny, brittle solids with intermediate to relatively good electrical. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. metalloids usually look like metals but behave largely like nonmetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semi. Arsenic,. Are Metalloids Lustrous Or Dull.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Are Metalloids Lustrous Or Dull Physically, they are shiny, brittle solids with intermediate to relatively good electrical. — metalloids can appear either lustrous or dull, demonstrating a range in physical appearance. Arsenic, a metalloid, exhibits a metallic luster, unlike. metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle. Some. Are Metalloids Lustrous Or Dull.