Chemistry Titration Ratio . — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its.
from saylordotorg.github.io
6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
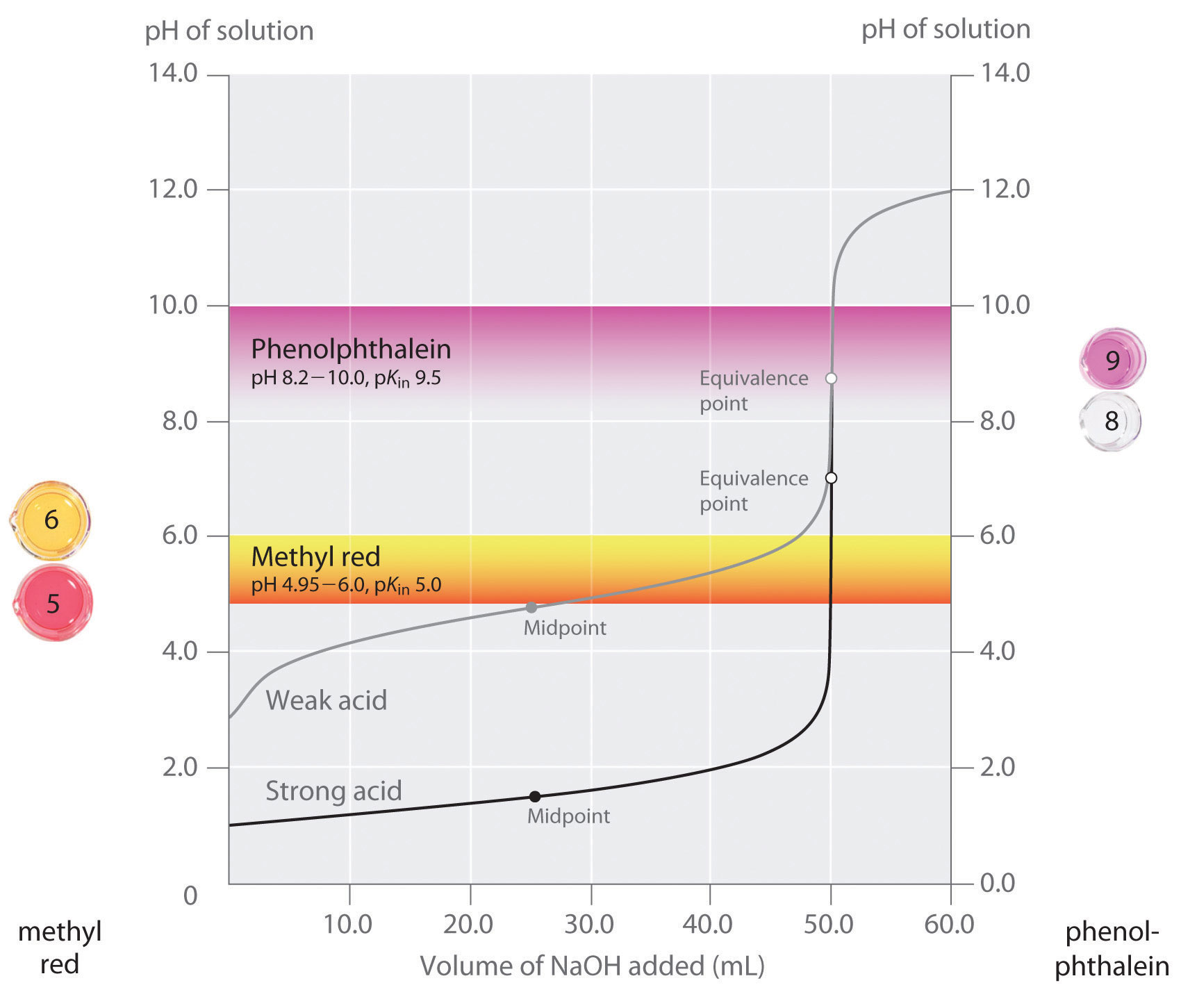
AcidBase Titrations
Chemistry Titration Ratio a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be.
From parholikhotoacknoledge.blogspot.com
Titrations in Chemistry Unveiling the Secrets of Quantitative Analysis Chemistry Titration Ratio the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another. Chemistry Titration Ratio.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Chemistry Titration Ratio a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration involves the gradual addition of a reagent of known concentration,. Chemistry Titration Ratio.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Chemistry Titration Ratio — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Chemistry Titration Ratio.
From saylordotorg.github.io
AcidBase Titrations Chemistry Titration Ratio — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. the above equation works only for neutralizations in which there is a 1:1 ratio between. Chemistry Titration Ratio.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. — titration calculations for a neutralization reaction with a. Chemistry Titration Ratio.
From www.scribd.com
Titration PDF Titration Chemistry Chemistry Titration Ratio Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 6.2 general principles and terms of titration processes a titration is the process of determining. Chemistry Titration Ratio.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts Chemistry Titration Ratio — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid. Chemistry Titration Ratio.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Chemistry Titration Ratio a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. — titration calculations for a neutralization reaction with a 1:1. Chemistry Titration Ratio.
From www.researchgate.net
Stoichiometric ratios determined by spectrophotometric titrations Chemistry Titration Ratio Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — a titration is a laboratory technique used to precisely measure molar concentration of. Chemistry Titration Ratio.
From www.reddit.com
[Grade 11 chemistry titration] Titratiok curve with two equivalence Chemistry Titration Ratio — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and.. Chemistry Titration Ratio.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and.. Chemistry Titration Ratio.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is an experiment where a volume of a solution of known concentration is added to a volume of. Chemistry Titration Ratio.
From learningcampusunpen.z13.web.core.windows.net
How To Calculate Mole Ratio In Chemistry Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — a titration is a laboratory technique used to precisely measure molar concentration of. Chemistry Titration Ratio.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Chemistry Titration Ratio — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is an experiment where a volume of a solution of known concentration is added to a volume. Chemistry Titration Ratio.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Chemistry Titration Ratio Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the. Chemistry Titration Ratio.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to. Chemistry Titration Ratio.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Chemistry Titration Ratio — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. the above equation works only for neutralizations in which. Chemistry Titration Ratio.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Chemistry Titration Ratio a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — a titration is a laboratory technique used to. Chemistry Titration Ratio.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Chemistry Titration Ratio — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. Titration involves the gradual addition of a reagent of known concentration, known as the titrant,. Chemistry Titration Ratio.
From learningschoolmysticis9.z22.web.core.windows.net
How To Do Titrations In Chemistry Chemistry Titration Ratio the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Chemistry Titration Ratio.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is an experiment where a volume of a solution of known concentration is. Chemistry Titration Ratio.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is an experiment where a volume of a solution of known concentration is. Chemistry Titration Ratio.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Chemistry Titration Ratio — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is an experiment where a volume of a solution of known concentration is added to a volume of. Chemistry Titration Ratio.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Chemistry Titration Ratio the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. 6.2 general principles and terms of titration processes a titration is the process of determining. Chemistry Titration Ratio.
From labthink.netlify.app
What is titration and how does it work Chemistry Titration Ratio — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. Titration involves the gradual addition of a reagent of known concentration, known as the titrant,. Chemistry Titration Ratio.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Chemistry Titration Ratio — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and.. Chemistry Titration Ratio.
From mavink.com
Titration Labeled Chemistry Titration Ratio a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. the above equation works only for neutralizations in. Chemistry Titration Ratio.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is an experiment where a volume of a solution of known concentration. Chemistry Titration Ratio.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Chemistry Titration Ratio Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. 6.2 general principles and terms of titration processes a titration is the process of. Chemistry Titration Ratio.
From pubs.rsc.org
A convenient chemical titration method to measure M( ii )/M( iii Chemistry Titration Ratio Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. 6.2 general principles and terms of titration processes a titration is the process of. Chemistry Titration Ratio.
From printablevozljevrc.z21.web.core.windows.net
How To Do Titrations In Chemistry Chemistry Titration Ratio the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and.. Chemistry Titration Ratio.
From revisechemistry.uk
Chemical Calculations Edexcel T1 revisechemistry.uk Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is an experiment where a volume of a solution of known concentration. Chemistry Titration Ratio.
From saylordotorg.github.io
AcidBase Titrations Chemistry Titration Ratio 6.2 general principles and terms of titration processes a titration is the process of determining the quantity of a substance a. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid. Chemistry Titration Ratio.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Chemistry Titration Ratio the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is an experiment where a volume of a solution of known concentration is. Chemistry Titration Ratio.
From theedge.com.hk
Chemistry How To Titration The Edge Chemistry Titration Ratio Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. — a titration is a laboratory technique used to precisely measure molar concentration of an. Chemistry Titration Ratio.