The Number Of Electrons In An Atom Is Equal To . learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. For example, a lithium atom (z=3, a=7 amu). The number of protons and. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom.
from wou.edu
For example, a lithium atom (z=3, a=7 amu). given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. The number of protons and. learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number.
CH150 Chapter 2 Atoms and Periodic Table Chemistry
The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. For example, a lithium atom (z=3, a=7 amu). learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. The number of protons and. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in. The Number Of Electrons In An Atom Is Equal To.
From www.slideserve.com
PPT Summary of the Atom PowerPoint Presentation ID350838 The Number Of Electrons In An Atom Is Equal To The number of protons and. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. learn how to use the periodic table and. The Number Of Electrons In An Atom Is Equal To.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table The Number Of Electrons In An Atom Is Equal To learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. learn how to use the periodic table and the atomic. The Number Of Electrons In An Atom Is Equal To.
From awesomehome.co
Using The Periodic Table To Determine Protons Neutrons And Electrons The Number Of Electrons In An Atom Is Equal To learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. For example, a lithium atom (z=3, a=7 amu). learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. learn about protons, neutrons and electrons, and how. The Number Of Electrons In An Atom Is Equal To.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry The Number Of Electrons In An Atom Is Equal To given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. learn how to find the number of protons, neutrons, and electrons of any element using the. The Number Of Electrons In An Atom Is Equal To.
From www.animalia-life.club
Electrons In An Atom The Number Of Electrons In An Atom Is Equal To given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. For example, a lithium atom (z=3, a=7 amu). learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. learn about protons, neutrons and electrons, and how. The Number Of Electrons In An Atom Is Equal To.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons And Electrons Elcho Table The Number Of Electrons In An Atom Is Equal To a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. learn how to find and calculate the. The Number Of Electrons In An Atom Is Equal To.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology The Number Of Electrons In An Atom Is Equal To a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. For example, a lithium atom (z=3, a=7 amu). learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions.. The Number Of Electrons In An Atom Is Equal To.
From circuitugandanur.z21.web.core.windows.net
Orbital Diagram Of Elements The Number Of Electrons In An Atom Is Equal To learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location. The Number Of Electrons In An Atom Is Equal To.
From www.pinterest.com
3 Ways to Calculate Atomic Mass wikiHow Teaching chemistry The Number Of Electrons In An Atom Is Equal To The number of protons and. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. . The Number Of Electrons In An Atom Is Equal To.
From schematicfixcinnamon.z5.web.core.windows.net
Diagram Of Protons Neutrons And Electrons The Number Of Electrons In An Atom Is Equal To learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. learn how to use the. The Number Of Electrons In An Atom Is Equal To.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts The Number Of Electrons In An Atom Is Equal To learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. learn how to use the periodic table and the atomic. The Number Of Electrons In An Atom Is Equal To.
From quizzmagicsims101.z21.web.core.windows.net
How To Work Out Protons Neutrons Electrons The Number Of Electrons In An Atom Is Equal To learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in. The Number Of Electrons In An Atom Is Equal To.
From www.vrogue.co
How To Calculate The Number Of Protons Neutrons And E vrogue.co The Number Of Electrons In An Atom Is Equal To learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location. The Number Of Electrons In An Atom Is Equal To.
From www.slideserve.com
PPT ATOMIC STRUCTURE PowerPoint Presentation, free download ID357852 The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. The number of protons and. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. . The Number Of Electrons In An Atom Is Equal To.
From www.britannica.com
Periodic table Elements, Groups, Properties Britannica The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. The number of protons and. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. . The Number Of Electrons In An Atom Is Equal To.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples The Number Of Electrons In An Atom Is Equal To learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. For example, a lithium atom (z=3, a=7 amu). The number of protons and. learn how to find. The Number Of Electrons In An Atom Is Equal To.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation ID1680708 The Number Of Electrons In An Atom Is Equal To learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. The number of protons and. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. . The Number Of Electrons In An Atom Is Equal To.
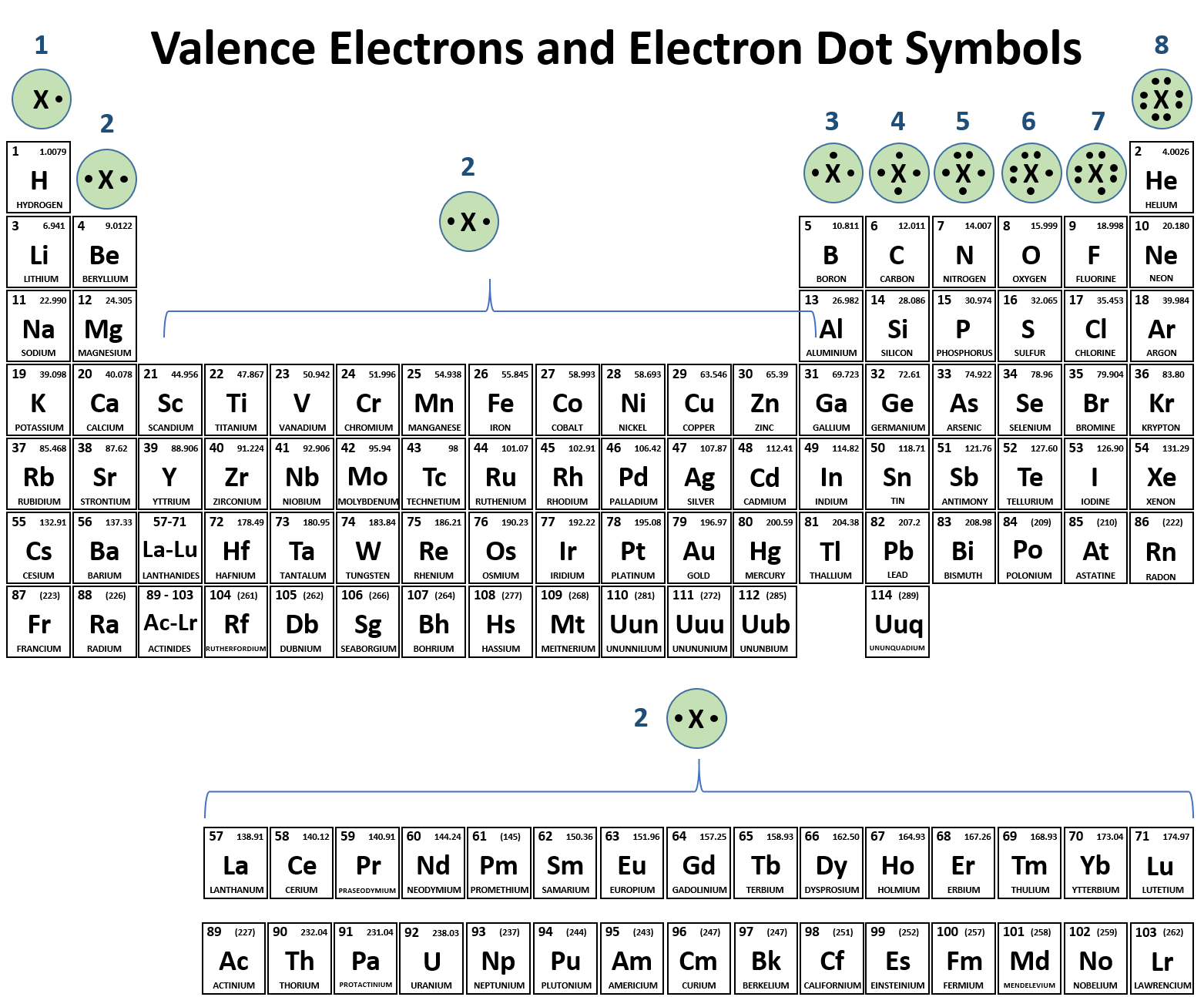
From enginedbconvectors.z22.web.core.windows.net
Lewis Electron Dot Structure Periodic Table The Number Of Electrons In An Atom Is Equal To learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. The number of protons and. learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. given an atomic number (z) and mass number (a), you. The Number Of Electrons In An Atom Is Equal To.
From frecetovbpschematic.z4.web.core.windows.net
Simple Diagram Of An Atom The Number Of Electrons In An Atom Is Equal To The number of protons and. learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. given an atomic number (z). The Number Of Electrons In An Atom Is Equal To.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation ID5055482 The Number Of Electrons In An Atom Is Equal To learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. The number of protons and. learn how to use. The Number Of Electrons In An Atom Is Equal To.
From howtodiscuss.com
Valence Electron How To Discuss The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. given an atomic number (z). The Number Of Electrons In An Atom Is Equal To.
From periodictable.me
Atomic Number Examples Archives Dynamic Periodic Table of Elements The Number Of Electrons In An Atom Is Equal To given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. learn how to use the periodic table and the atomic weight to calculate. The Number Of Electrons In An Atom Is Equal To.
From www.periodic-table.org
What is Atomic and Nuclear Structure Definition The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. The number of protons and. learn about protons, neutrons and. The Number Of Electrons In An Atom Is Equal To.
From circuitwiringpapes55.z22.web.core.windows.net
Diagram Of Protons Neutrons And Electrons The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. learn how to find the number of protons,. The Number Of Electrons In An Atom Is Equal To.
From studylib.net
atom The Number Of Electrons In An Atom Is Equal To learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. For example, a lithium atom (z=3, a=7 amu).. The Number Of Electrons In An Atom Is Equal To.
From futuresoftech.com
what is the atomic number The Number Of Electrons In An Atom Is Equal To The number of protons and. For example, a lithium atom (z=3, a=7 amu). learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. learn how. The Number Of Electrons In An Atom Is Equal To.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. a neutral atom must contain the same number. The Number Of Electrons In An Atom Is Equal To.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow The Number Of Electrons In An Atom Is Equal To learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. learn how to find and calculate the number of protons, neutrons, and electrons in an. The Number Of Electrons In An Atom Is Equal To.
From diagramthefactualist09.z21.web.core.windows.net
How To Determine Charge Of Atom The Number Of Electrons In An Atom Is Equal To For example, a lithium atom (z=3, a=7 amu). learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. given an atomic number (z). The Number Of Electrons In An Atom Is Equal To.
From www.broadlearnings.com
Atomic Structure Broad Learnings The Number Of Electrons In An Atom Is Equal To learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. For example, a lithium atom (z=3,. The Number Of Electrons In An Atom Is Equal To.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica The Number Of Electrons In An Atom Is Equal To a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number. learn how to use the periodic table and the atomic weight to calculate the number of protons, neutrons and electrons in any element. The number of protons and. learn about protons, neutrons and electrons, and how. The Number Of Electrons In An Atom Is Equal To.
From www.vrogue.co
How To Calculate The Number Of Protons Neutrons And E vrogue.co The Number Of Electrons In An Atom Is Equal To learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. For example, a lithium atom (z=3, a=7 amu). learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. learn. The Number Of Electrons In An Atom Is Equal To.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic The Number Of Electrons In An Atom Is Equal To learn about protons, neutrons and electrons, and how to calculate their relative mass, charge and location in the atom. given an atomic number (z) and mass number (a), you can find the number of protons, neutrons, and electrons in a neutral atom. a neutral atom must contain the same number of positive and negative charges, so the. The Number Of Electrons In An Atom Is Equal To.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol The Number Of Electrons In An Atom Is Equal To learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. learn how to find the number of protons, neutrons, and electrons of any element using the periodic table, the atomic number, the mass number, and the charge of ions. learn about protons, neutrons and electrons, and how to calculate. The Number Of Electrons In An Atom Is Equal To.