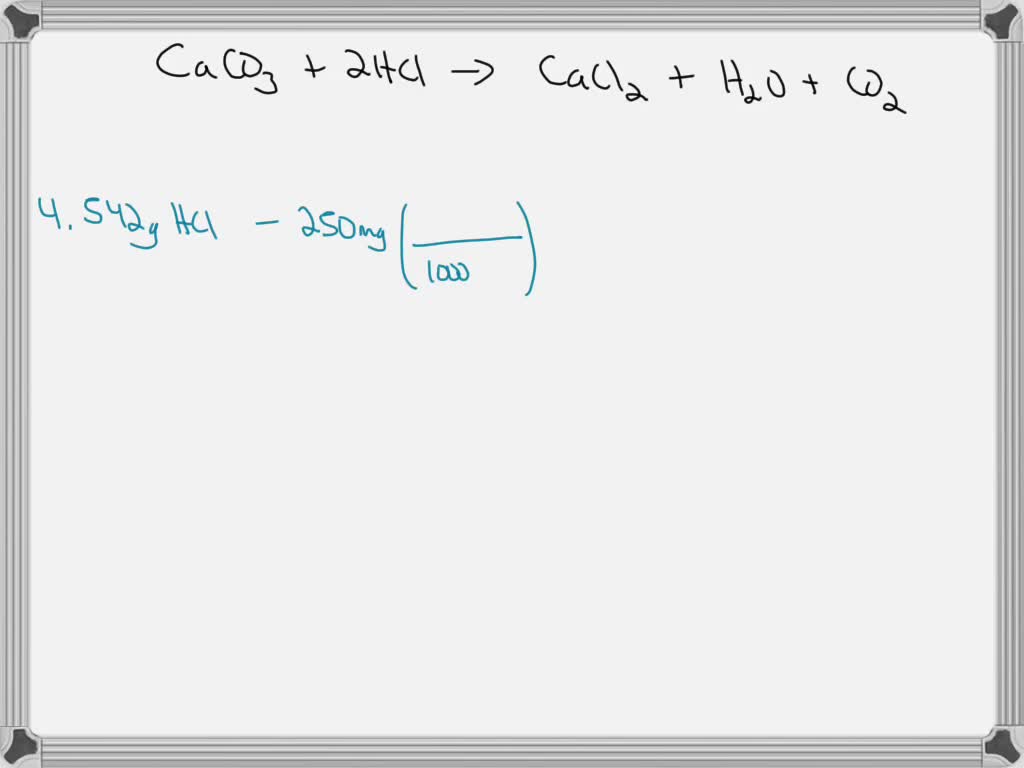How Do Calcium Carbonate Antacids Work . Calcium carbonate works in the small intestines as a phosphate binder. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. This acid and base react. As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. Antacids work by neutralizing stomach acid. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. They do this through a chemical reaction in which the antacid combines with the acid in the. What are antacids and how do they work? They do this because the chemicals in antacids are bases. Antacids work by counteracting (neutralising) the acid in your stomach. Most antacids work because they contain at least one of these key ingredients: The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms.
from www.numerade.com
The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. Antacids work by counteracting (neutralising) the acid in your stomach. Most antacids work because they contain at least one of these key ingredients: Calcium carbonate works in the small intestines as a phosphate binder. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. Antacids work by neutralizing stomach acid. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. They do this through a chemical reaction in which the antacid combines with the acid in the. As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis.
SOLVED Calcium carbonate (CaCO3) is a common ingredient in antacids
How Do Calcium Carbonate Antacids Work They do this through a chemical reaction in which the antacid combines with the acid in the. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. Antacids work by neutralizing stomach acid. What are antacids and how do they work? Calcium carbonate, magnesium hydroxide, aluminum hydroxide. Antacids work by counteracting (neutralising) the acid in your stomach. Most antacids work because they contain at least one of these key ingredients: They do this because the chemicals in antacids are bases. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. This acid and base react. Calcium carbonate works in the small intestines as a phosphate binder. They do this through a chemical reaction in which the antacid combines with the acid in the. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid.
From www.walmart.com
Equate UltraStrength Antacid Tablets, Calcium Carbonate, 1000 mg How Do Calcium Carbonate Antacids Work They do this through a chemical reaction in which the antacid combines with the acid in the. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. Antacids work by counteracting (neutralising) the acid in your stomach. This acid and base react. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. As an antacid, calcium. How Do Calcium Carbonate Antacids Work.
From www.chegg.com
Solved Calcium carbonate is a common ingredient in antacids How Do Calcium Carbonate Antacids Work They do this through a chemical reaction in which the antacid combines with the acid in the. This acid and base react. Antacids work by neutralizing stomach acid. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. Antacids work by counteracting (neutralising) the acid in your. How Do Calcium Carbonate Antacids Work.
From ar.inspiredpencil.com
Calcium Carbonate Supplement How Do Calcium Carbonate Antacids Work They do this through a chemical reaction in which the antacid combines with the acid in the. Antacids work by counteracting (neutralising) the acid in your stomach. Antacids work by neutralizing stomach acid. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. This acid and base react. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the. How Do Calcium Carbonate Antacids Work.
From www.kroger.com
Antacids Alkums Calcium Carbonate Antacid, 750 mg Chewable Tablet, 96 How Do Calcium Carbonate Antacids Work Most antacids work because they contain at least one of these key ingredients: Calcium carbonate works in the small intestines as a phosphate binder. This acid and base react. Antacids work by counteracting (neutralising) the acid in your stomach. They do this through a chemical reaction in which the antacid combines with the acid in the. They do this because. How Do Calcium Carbonate Antacids Work.
From exoxxtand.blob.core.windows.net
How Do Calcium Carbonate Shells Form at Dawn Fisher blog How Do Calcium Carbonate Antacids Work As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. Calcium carbonate works in the small intestines as a phosphate binder. They do this because the chemicals in antacids are bases. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. What are antacids and how do they work? Stomach acid is essential for digesting food and killing bacteria, but too much. How Do Calcium Carbonate Antacids Work.
From www.numerade.com
SOLVED Calcium carbonate (CaCO3) is a common ingredient in antacids How Do Calcium Carbonate Antacids Work What are antacids and how do they work? Calcium carbonate, magnesium hydroxide, aluminum hydroxide. Most antacids work because they contain at least one of these key ingredients: Antacids work by neutralizing stomach acid. They do this because the chemicals in antacids are bases. Antacids work by counteracting (neutralising) the acid in your stomach. Calcium carbonate works in the small intestines. How Do Calcium Carbonate Antacids Work.
From www.chemist-4-u.com
Buy Calcium Carbonate Antacids for Heartburn Chemist 4 U How Do Calcium Carbonate Antacids Work Antacids work by neutralizing stomach acid. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. Most antacids work because they. How Do Calcium Carbonate Antacids Work.
From www.desertcart.ae
Buy Tums Ant/Calcium Supplement, Extra Strength 750, Chewable s How Do Calcium Carbonate Antacids Work Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. They do this through a chemical reaction in which the antacid combines with the acid in the. What are antacids and how do they work? Antacids work by counteracting (neutralising) the acid in your stomach. Most antacids work because they contain at. How Do Calcium Carbonate Antacids Work.
From health.gov.capital
How do antacids work to relieve digestive issues? Health.Gov.Capital How Do Calcium Carbonate Antacids Work Most antacids work because they contain at least one of these key ingredients: As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. They do this because the chemicals in antacids are bases. Antacids work by neutralizing stomach acid. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn. How Do Calcium Carbonate Antacids Work.
From www.heb.com
HEB Calcium Carbonate Antacid Tablets 750 mg Shop Digestion How Do Calcium Carbonate Antacids Work They do this because the chemicals in antacids are bases. This acid and base react. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. They do this through a chemical reaction in which the antacid combines with the acid in. How Do Calcium Carbonate Antacids Work.
From www.britannica.com
How Do Antacids Work? Britannica How Do Calcium Carbonate Antacids Work This acid and base react. Antacids work by counteracting (neutralising) the acid in your stomach. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. Most. How Do Calcium Carbonate Antacids Work.
From www.3benefitsof.com
Antacids 101 Benefits & Important Things to Know 3 Benefits Of How Do Calcium Carbonate Antacids Work Antacids work by neutralizing stomach acid. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. Antacids work by counteracting (neutralising) the acid in your stomach. They do this through a chemical reaction in. How Do Calcium Carbonate Antacids Work.
From www.alamy.com
Calcium carbonate molecule formula and 3D diagram. Digital illustration How Do Calcium Carbonate Antacids Work The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. What are antacids and how do they work? Calcium carbonate works in the small intestines as a phosphate binder. Antacids work by neutralizing stomach acid. The active ingredient in many antacids is calcium carbonate. How Do Calcium Carbonate Antacids Work.
From www.gnhindia.com
BUY Calcium Carbonate Antacid) 1000 mg/1 from GNH India at How Do Calcium Carbonate Antacids Work Antacids work by counteracting (neutralising) the acid in your stomach. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. Antacids work by neutralizing stomach acid. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several. How Do Calcium Carbonate Antacids Work.
From slsi.lk
slsi.lk how long for sulfatrim to work Something is. calcium How Do Calcium Carbonate Antacids Work Antacids work by counteracting (neutralising) the acid in your stomach. As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. This acid and base react. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. They do this through a chemical reaction in which the antacid combines with the acid. How Do Calcium Carbonate Antacids Work.
From www.slideserve.com
PPT How do Antacids Work? PowerPoint Presentation, free download ID How Do Calcium Carbonate Antacids Work Calcium carbonate, magnesium hydroxide, aluminum hydroxide. As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. This acid and base react. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. Calcium carbonate works in the small intestines as a phosphate binder. They. How Do Calcium Carbonate Antacids Work.
From www.youtube.com
1. Calcium Carbonate (Children's Pepto) Which antacid works best How Do Calcium Carbonate Antacids Work Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. What are antacids and how do they work? As an antacid, calcium carbonate also increases gastrointestinal motility and initiates. How Do Calcium Carbonate Antacids Work.
From www.haikudeck.com
Calcium Carbonate by Harsimar How Do Calcium Carbonate Antacids Work This acid and base react. Calcium carbonate works in the small intestines as a phosphate binder. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. What are antacids and how do they work? Most antacids work because they contain at least one of. How Do Calcium Carbonate Antacids Work.
From www.slideshare.net
Antacids How Do Calcium Carbonate Antacids Work Antacids work by neutralizing stomach acid. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. Calcium carbonate works in the small intestines as a phosphate binder. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in. How Do Calcium Carbonate Antacids Work.
From www.chemist-4-u.com
Buy Calcium Carbonate Antacids for Heartburn Chemist 4 U How Do Calcium Carbonate Antacids Work Calcium carbonate works in the small intestines as a phosphate binder. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. They do this because the chemicals in antacids are bases. What are antacids and how do they work? Stomach acid is essential for digesting food and killing bacteria, but too much. How Do Calcium Carbonate Antacids Work.
From www.bobbarker.com
Calcium Carbonate Antacid How Do Calcium Carbonate Antacids Work They do this because the chemicals in antacids are bases. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. Stomach acid is essential for digesting. How Do Calcium Carbonate Antacids Work.
From journals.sagepub.com
Antacids revisited review on contemporary facts and relevance for self How Do Calcium Carbonate Antacids Work As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. They do this because the chemicals in antacids are bases. This acid and base react. Antacids work by counteracting (neutralising) the acid in your stomach.. How Do Calcium Carbonate Antacids Work.
From www.slideserve.com
PPT How do Antacids Work? PowerPoint Presentation, free download ID How Do Calcium Carbonate Antacids Work Most antacids work because they contain at least one of these key ingredients: As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. They do this because the chemicals in antacids are. How Do Calcium Carbonate Antacids Work.
From dailymed.nlm.nih.gov
DailyMed CARE ONE CALCIUM ANTACID calcium carbonate tablet, chewable How Do Calcium Carbonate Antacids Work Calcium carbonate works in the small intestines as a phosphate binder. They do this through a chemical reaction in which the antacid combines with the acid in the. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is. How Do Calcium Carbonate Antacids Work.
From www.numerade.com
SOLVED Antacids are used to treat acid reflux by neutralizing stomach How Do Calcium Carbonate Antacids Work Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. They do this because the chemicals in antacids are bases. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. Antacids work by counteracting (neutralising) the acid in your stomach. They do this through a chemical reaction in which the antacid combines with the acid in. How Do Calcium Carbonate Antacids Work.
From slsi.lk
slsi.lk how long for sulfatrim to work What is calcium carbonate How Do Calcium Carbonate Antacids Work They do this through a chemical reaction in which the antacid combines with the acid in the. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. The calcium. How Do Calcium Carbonate Antacids Work.
From www.walmart.com
KS Ultra Strength Antacid Calcium Carbonate 1000 MG Assorted Berry How Do Calcium Carbonate Antacids Work As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. Calcium carbonate, magnesium hydroxide, aluminum hydroxide. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and. How Do Calcium Carbonate Antacids Work.
From www.numerade.com
SOLVED Calcium carbonate (CaCO3) is a common ingredient in antacids How Do Calcium Carbonate Antacids Work Antacids work by neutralizing stomach acid. What are antacids and how do they work? Calcium carbonate, magnesium hydroxide, aluminum hydroxide. This acid and base react. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. Most antacids work because they contain at least one of. How Do Calcium Carbonate Antacids Work.
From www.wikidoc.org
Calcium carbonate wikidoc How Do Calcium Carbonate Antacids Work Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. Calcium carbonate works in the small intestines as a phosphate binder. They do this through a chemical. How Do Calcium Carbonate Antacids Work.
From slsi.lk
slsi.lk how long for sulfatrim to work How many calcium carbonate How Do Calcium Carbonate Antacids Work Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. Calcium carbonate works in the small intestines as a phosphate binder. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. Calcium carbonate, magnesium hydroxide, aluminum hydroxide.. How Do Calcium Carbonate Antacids Work.
From exoxxtand.blob.core.windows.net
How Do Calcium Carbonate Shells Form at Dawn Fisher blog How Do Calcium Carbonate Antacids Work Antacids work by counteracting (neutralising) the acid in your stomach. Calcium carbonate works in the small intestines as a phosphate binder. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. They do this because the chemicals in antacids are bases. Calcium carbonate, magnesium. How Do Calcium Carbonate Antacids Work.
From www.smithsfoodanddrug.com
Antacids Alkums Calcium Carbonate Antacid, 750 mg Chewable Tablet, 96 How Do Calcium Carbonate Antacids Work As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. This acid and base react. Antacids work by neutralizing stomach acid. Ph is a measure that indicates the concentration of hydrogen ions. How Do Calcium Carbonate Antacids Work.
From www.walmart.com
Calcium Carbonate Oral Suspension Antacid 1250mg, Sugar & AlcoholFree How Do Calcium Carbonate Antacids Work Most antacids work because they contain at least one of these key ingredients: The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. This acid and base react. Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach. How Do Calcium Carbonate Antacids Work.
From www.peptest.co.nz
Alginate Antacids for Treating Reflux A Study Peptest Peptest How Do Calcium Carbonate Antacids Work Ph is a measure that indicates the concentration of hydrogen ions in a solution, like your stomach acid. This acid and base react. As an antacid, calcium carbonate also increases gastrointestinal motility and initiates peristalsis. Stomach acid is essential for digesting food and killing bacteria, but too much of it can lead to heartburn and other uncomfortable symptoms. They do. How Do Calcium Carbonate Antacids Work.
From medcline.eu
Acid Reflux Medicine (What They Are & Side Effects) MedCline How Do Calcium Carbonate Antacids Work Calcium carbonate, magnesium hydroxide, aluminum hydroxide. The active ingredient in many antacids is calcium carbonate (caco₃), a base that is actually found in several natural minerals, including limestone, marble, and chalk. The calcium released from calcium carbonate is known to increase peristalsis in the esophagus, pushing the acid into the stomach and providing relief from heartburn symptoms. Stomach acid is. How Do Calcium Carbonate Antacids Work.