Calorimeter Heat Capacity Equation . then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. use equation 5 to calculate the specific heat capacity of the unknown metal. Cs metal = equation 5 = mass of water in. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Calculate and interpret heat and related properties using typical calorimetry data. this equation binds temperature change and heat: Explain the technique of calorimetry. if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter.
from www.slideshare.net
Cs metal = equation 5 = mass of water in. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). use equation 5 to calculate the specific heat capacity of the unknown metal. if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. this equation binds temperature change and heat:
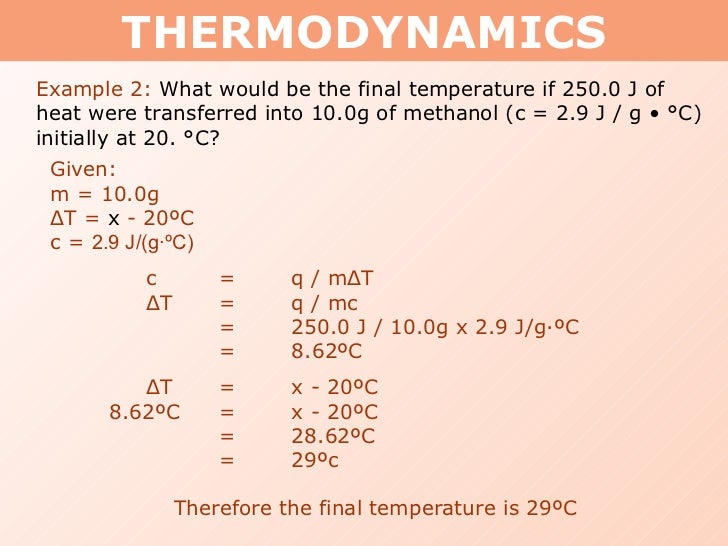
Tang 01 heat capacity and calorimetry
Calorimeter Heat Capacity Equation in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). this equation binds temperature change and heat: Cs metal = equation 5 = mass of water in. use equation 5 to calculate the specific heat capacity of the unknown metal. if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\).
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimeter Heat Capacity Equation Explain the technique of calorimetry. Cs metal = equation 5 = mass of water in. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calculate and interpret heat and related properties using typical calorimetry data. use equation 5 to. Calorimeter Heat Capacity Equation.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Heat Capacity Equation then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. Calculate and interpret heat and related properties using typical calorimetry data. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). this equation binds temperature. Calorimeter Heat Capacity Equation.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Calorimeter Heat Capacity Equation Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the. Calorimeter Heat Capacity Equation.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimeter Heat Capacity Equation use equation 5 to calculate the specific heat capacity of the unknown metal. if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta. Calorimeter Heat Capacity Equation.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calorimeter Heat Capacity Equation q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter. then use equation \ref{12.3.1}. Calorimeter Heat Capacity Equation.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Heat Capacity Equation use equation 5 to calculate the specific heat capacity of the unknown metal. this equation binds temperature change and heat: then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. Calculate and interpret heat and related properties using typical calorimetry data. Cs metal = equation 5 = mass of water. Calorimeter Heat Capacity Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimeter Heat Capacity Equation use equation 5 to calculate the specific heat capacity of the unknown metal. Calculate and interpret heat and related properties using typical calorimetry data. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is. Calorimeter Heat Capacity Equation.
From studylib.net
Heat Equation Calorimeter Heat Capacity Equation \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). this equation binds temperature change and heat: Explain the technique of calorimetry. then use equation \ref{12.3.1} to determine the heat capacity of the. Calorimeter Heat Capacity Equation.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Heat Capacity Equation then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. Calculate and interpret heat and related properties using typical calorimetry data. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. \scriptsize \delta. Calorimeter Heat Capacity Equation.
From exopfwwvc.blob.core.windows.net
Calorimeter In Physics Definition at Laura Addy blog Calorimeter Heat Capacity Equation Cs metal = equation 5 = mass of water in. use equation 5 to calculate the specific heat capacity of the unknown metal. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the. Calorimeter Heat Capacity Equation.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Heat Capacity Equation Calculate and interpret heat and related properties using typical calorimetry data. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. use equation 5 to calculate the specific heat capacity of the unknown metal. \scriptsize \delta q = m \cdot. Calorimeter Heat Capacity Equation.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Heat Capacity Equation this equation binds temperature change and heat: Explain the technique of calorimetry. use equation 5 to calculate the specific heat capacity of the unknown metal. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). \scriptsize \delta q = m \cdot c. Calorimeter Heat Capacity Equation.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Calorimeter Heat Capacity Equation Explain the technique of calorimetry. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. Cs metal = equation 5 = mass of water in. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. Calorimeter Heat Capacity Equation.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimeter Heat Capacity Equation then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). use equation 5 to calculate the specific heat capacity of the unknown metal. Explain the technique of calorimetry. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c. Calorimeter Heat Capacity Equation.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep Calorimeter Heat Capacity Equation q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. this equation binds temperature change and heat: then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. in the chapter on. Calorimeter Heat Capacity Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Heat Capacity Equation then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. Explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. if you wanted to use this whole. Calorimeter Heat Capacity Equation.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Heat Capacity Equation \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and. Calorimeter Heat Capacity Equation.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Heat Capacity Equation q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calculate and interpret heat and related properties using typical calorimetry data. Cs metal = equation 5 = mass of water in. if you wanted to use this whole formula for. Calorimeter Heat Capacity Equation.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube Calorimeter Heat Capacity Equation q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)). Calorimeter Heat Capacity Equation.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Heat Capacity Equation use equation 5 to calculate the specific heat capacity of the unknown metal. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. Cs metal = equation 5 = mass of water in.. Calorimeter Heat Capacity Equation.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Heat Capacity Equation q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter. Cs metal = equation 5. Calorimeter Heat Capacity Equation.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Calorimeter Heat Capacity Equation then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Explain the technique of calorimetry. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. Cs metal = equation 5 = mass of water in. in the chapter on temperature and heat, we defined. Calorimeter Heat Capacity Equation.
From printablelistquinta.z21.web.core.windows.net
How To Calculate For Heat Calorimeter Heat Capacity Equation then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. Explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of. Calorimeter Heat Capacity Equation.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimeter Heat Capacity Equation Explain the technique of calorimetry. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. \scriptsize \delta q = m \cdot c \cdot. Calorimeter Heat Capacity Equation.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The Calorimeter Heat Capacity Equation then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). this equation binds temperature change and heat: Calculate and interpret heat and related properties using. Calorimeter Heat Capacity Equation.
From users.highland.edu
Calorimetry Calorimeter Heat Capacity Equation Calculate and interpret heat and related properties using typical calorimetry data. Cs metal = equation 5 = mass of water in. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). this equation binds. Calorimeter Heat Capacity Equation.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Calorimeter Heat Capacity Equation then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. Calculate and interpret heat and related properties using typical calorimetry data. Cs metal = equation 5 = mass of water in. Explain the technique of calorimetry. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅. Calorimeter Heat Capacity Equation.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Heat Capacity Equation in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅. Calorimeter Heat Capacity Equation.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimeter Heat Capacity Equation Cs metal = equation 5 = mass of water in. this equation binds temperature change and heat: Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the. Calorimeter Heat Capacity Equation.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Heat Capacity Equation in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). Explain the technique of calorimetry. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. this equation binds temperature change and heat: Cs metal = equation. Calorimeter Heat Capacity Equation.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Heat Capacity Equation if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. Calculate and interpret heat and related properties using typical calorimetry data. \scriptsize \delta q = m. Calorimeter Heat Capacity Equation.
From www.slideserve.com
PPT CHEM 1011 PowerPoint Presentation, free download ID317228 Calorimeter Heat Capacity Equation in the chapter on temperature and heat, we defined the specific heat capacity with the equation \(q = mc\delta t\), or \(c = (1/m)q/\delta t\). q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. use equation 5 to. Calorimeter Heat Capacity Equation.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimeter Heat Capacity Equation q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Cs metal = equation 5 = mass of water in. Explain the technique of calorimetry. use equation 5 to calculate the specific heat capacity of the unknown metal. if. Calorimeter Heat Capacity Equation.
From chemistrytalk.org
Calorimetry ChemTalk Calorimeter Heat Capacity Equation \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb. if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the. Calorimeter Heat Capacity Equation.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , Calorimeter Heat Capacity Equation Explain the technique of calorimetry. \scriptsize \delta q = m \cdot c \cdot \delta t δq = m ⋅ c ⋅ δt. this equation binds temperature change and heat: if you wanted to use this whole formula for solving the calorimeter's specific heat capacity, you would need to know the mass of the calorimeter. then use equation. Calorimeter Heat Capacity Equation.