Iron Iii Chloride Charge . Some of the metals form very common ions which have latin names that are in. iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. 3.1 introduction to the octet rule. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. there are two ways to make this distinction. so fe +2 is iron(ii) and fe +3 is iron(iii). iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. For example, iron( ii ) has a. In the simpler, more modern approach, called the stock system, an ion’s positive charge. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. roman numeral notation indicates charge of ion when element commonly forms more than one ion.
from lab.honeywell.com
iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. there are two ways to make this distinction. roman numeral notation indicates charge of ion when element commonly forms more than one ion. Some of the metals form very common ions which have latin names that are in. In the simpler, more modern approach, called the stock system, an ion’s positive charge. For example, iron( ii ) has a. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron.
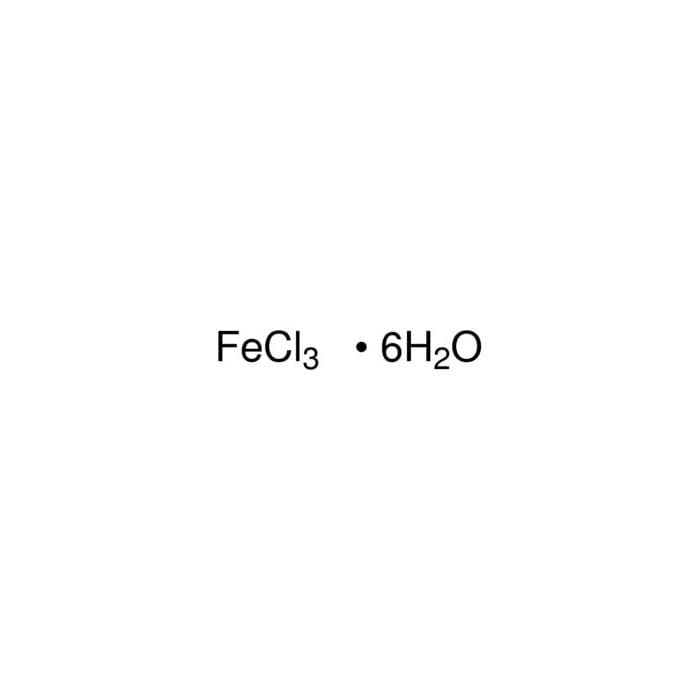
Iron(III) chloride hexahydrate 31232 Honeywell Research Chemicals
Iron Iii Chloride Charge 3.1 introduction to the octet rule. For example, iron( ii ) has a. so fe +2 is iron(ii) and fe +3 is iron(iii). iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. there are two ways to make this distinction. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. Some of the metals form very common ions which have latin names that are in. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. 3.1 introduction to the octet rule. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). roman numeral notation indicates charge of ion when element commonly forms more than one ion. In the simpler, more modern approach, called the stock system, an ion’s positive charge. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged.
From app.pandai.org
Chemical Formulae Iron Iii Chloride Charge Some of the metals form very common ions which have latin names that are in. there are two ways to make this distinction. roman numeral notation indicates charge of ion when element commonly forms more than one ion. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride,. Iron Iii Chloride Charge.
From dxogvupte.blob.core.windows.net
Iron (Iii) Symbol And Charge at Rocky Molina blog Iron Iii Chloride Charge so fe +2 is iron(ii) and fe +3 is iron(iii). iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). 3.1 introduction to the octet rule. In the simpler, more modern approach, called. Iron Iii Chloride Charge.
From apcpure.com
Iron (III) Chloride Anhydrous APC Pure Iron Iii Chloride Charge iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. so fe +2 is iron(ii) and fe +3 is iron(iii). iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. there are two ways to make this distinction. In the. Iron Iii Chloride Charge.
From www.fishersci.co.uk
Iron(III) chloride hexahydrate, 99+, for analysis, Thermo Scientific Iron Iii Chloride Charge In the simpler, more modern approach, called the stock system, an ion’s positive charge. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). 3.1 introduction to the octet rule. roman numeral notation indicates charge of ion when element commonly forms more than one ion. there are two. Iron Iii Chloride Charge.
From www.scientificlabs.co.uk
Iron(III) chloride, reagent gr 1577405G SIGMAALDRICH SLS Iron Iii Chloride Charge so fe +2 is iron(ii) and fe +3 is iron(iii). Some of the metals form very common ions which have latin names that are in. For example, iron( ii ) has a. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. 3.1 introduction to the octet rule. therefore, the iron. Iron Iii Chloride Charge.
From shopee.co.th
Iron(III) Chloride Hexahydrate Grade AR 1KG (QRec) Shopee Thailand Iron Iii Chloride Charge so fe +2 is iron(ii) and fe +3 is iron(iii). there are two ways to make this distinction. In the simpler, more modern approach, called the stock system, an ion’s positive charge. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). roman numeral notation indicates charge. Iron Iii Chloride Charge.
From www.selectschoolsupplies.co.uk
Iron (III) Chloride Anhydrous 250g Iron Iii Chloride Charge 3.1 introduction to the octet rule. there are two ways to make this distinction. In the simpler, more modern approach, called the stock system, an ion’s positive charge. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. so fe +2 is iron(ii) and fe +3. Iron Iii Chloride Charge.
From sciencephotogallery.com
Sodium Hydroxide And Iron IIi Chloride by Martyn F. Chillmaid/science Iron Iii Chloride Charge 3.1 introduction to the octet rule. there are two ways to make this distinction. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. For example, iron( ii ) has a. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in. Iron Iii Chloride Charge.
From www.libertysci.com
Iron(III) Chloride, hexahydrate, 25 g Liberty Scientific Iron Iii Chloride Charge For example, iron( ii ) has a. In the simpler, more modern approach, called the stock system, an ion’s positive charge. roman numeral notation indicates charge of ion when element commonly forms more than one ion. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. . Iron Iii Chloride Charge.
From www.homesciencetools.com
Iron(III) Chloride, 30 g Ferric Chloride Home Science Tools Iron Iii Chloride Charge 3.1 introduction to the octet rule. roman numeral notation indicates charge of ion when element commonly forms more than one ion. In the simpler, more modern approach, called the stock system, an ion’s positive charge. there are two ways to make this distinction. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the. Iron Iii Chloride Charge.
From molekula.com
Purchase Iron(III) chloride hexahydrate [10025771] online • Catalog Iron Iii Chloride Charge For example, iron( ii ) has a. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. Some of the metals form very common ions which have latin names that are in. so fe +2 is iron(ii) and fe +3 is. Iron Iii Chloride Charge.
From www.ibuychemikals.com
Buy Iron III chloride anhydrous LR 500 gm onlineibuychemikals Iron Iii Chloride Charge For example, iron( ii ) has a. 3.1 introduction to the octet rule. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. iron (iii). Iron Iii Chloride Charge.
From www.fishersci.se
Iron(III) chloride, 98, pure, anhydrous, Thermo Scientific Chemicals Iron Iii Chloride Charge so fe +2 is iron(ii) and fe +3 is iron(iii). iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a. Up until now we have been discussing only the elemental. Iron Iii Chloride Charge.
From www.slideserve.com
PPT Review of Atomic Model PowerPoint Presentation, free download Iron Iii Chloride Charge For example, iron( ii ) has a. roman numeral notation indicates charge of ion when element commonly forms more than one ion. so fe +2 is iron(ii) and fe +3 is iron(iii). therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge. Iron Iii Chloride Charge.
From lab.honeywell.com
Iron(III) chloride hexahydrate 31232 Honeywell Research Chemicals Iron Iii Chloride Charge For example, iron( ii ) has a. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. This is because. Iron Iii Chloride Charge.
From www.sigmaaldrich.co.th
IRON(III) CHLORIDE, 97 Merck Life Sciences Thailand Iron Iii Chloride Charge there are two ways to make this distinction. For example, iron( ii ) has a. In the simpler, more modern approach, called the stock system, an ion’s positive charge. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. This is because the number of electrons (negative. Iron Iii Chloride Charge.
From testbook.com
Iron (III) Chloride Formula Know Structure, Preparation,& Uses Iron Iii Chloride Charge iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. roman numeral notation indicates charge of ion when element commonly forms more than one ion. In the simpler, more modern approach, called the stock system, an ion’s positive charge. For example, iron( ii ) has a. there are two ways to make. Iron Iii Chloride Charge.
From www.slideserve.com
PPT Chemical Nomenclature (Naming Compounds) PowerPoint Presentation Iron Iii Chloride Charge therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. there are two ways to make this distinction. iron(iii) chloride, chemically represented as. Iron Iii Chloride Charge.
From hoachatthinghiem.org
Iron (III) Chloride Hexahydrate (FeCl3.6H2O, AR, Chai 500G, Xilong, Cas Iron Iii Chloride Charge 3.1 introduction to the octet rule. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. so fe +2 is iron(ii) and fe +3. Iron Iii Chloride Charge.
From www.shutterstock.com
Iron Iii Chloride Handwritten Chemical Formula Stock Illustration Iron Iii Chloride Charge so fe +2 is iron(ii) and fe +3 is iron(iii). therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. For example, iron( ii ). Iron Iii Chloride Charge.
From www.youtube.com
Equation for FeCl3 + H2O Iron (III) chloride + Water YouTube Iron Iii Chloride Charge iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. 3.1 introduction to the octet rule. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. Some of the metals form very common ions which have latin names that are in. . Iron Iii Chloride Charge.
From 1malaysiabiolab.com
Iron (III) Chloride Hexahydrate 1Malaysia Bio Lab Iron Iii Chloride Charge 3.1 introduction to the octet rule. iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. there are two ways to make this distinction. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and hydrogen chloride, or iron. so fe +2 is iron(ii) and. Iron Iii Chloride Charge.
From www.youtube.com
Lewis Structure of Iron (III) Chloride, FeCl3 YouTube Iron Iii Chloride Charge Some of the metals form very common ions which have latin names that are in. 3.1 introduction to the octet rule. roman numeral notation indicates charge of ion when element commonly forms more than one ion. there are two ways to make this distinction. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and. Iron Iii Chloride Charge.
From www.youtube.com
How to Balance FeCl3 + Na2S = Fe2S3 + NaCl (Iron (III) chloride Iron Iii Chloride Charge there are two ways to make this distinction. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. so fe +2 is iron(ii). Iron Iii Chloride Charge.
From pubs.acs.org
Revisiting the Determination of Percent Aspirin Lab Using a Limiting Iron Iii Chloride Charge so fe +2 is iron(ii) and fe +3 is iron(iii). In the simpler, more modern approach, called the stock system, an ion’s positive charge. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the. Iron Iii Chloride Charge.
From www.indiamart.com
Iron(Iii)Chloride, FeCl3 at best price in Vadodara ID 18993008712 Iron Iii Chloride Charge therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. there are two ways to make this distinction. iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. This is because the number of electrons. Iron Iii Chloride Charge.
From www.npchem.co.th
IRON (III) CHLORIDE anhydrous, Lab, 500G, ยี่ห้อ ChemSupply Iron Iii Chloride Charge Some of the metals form very common ions which have latin names that are in. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). For example, iron( ii ) has a. iron (iii) chloride hydrates cannot be dehydrated to anhydrous, because it decomposes to iron (iii) oxychloride and. Iron Iii Chloride Charge.
From sochemvn.com
Iron (III) Chloride Solution FeCl3 40 HÓA CHẤT CƠ BẢN MIỀN NAM Iron Iii Chloride Charge In the simpler, more modern approach, called the stock system, an ion’s positive charge. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. For example, iron( ii ) has a. so fe +2 is iron(ii) and fe +3 is iron(iii).. Iron Iii Chloride Charge.
From www.tokopedia.com
Jual Iron(III) Chloride hexahydrate (FeCl3.6H2O), 250 Gr, MERCK, 103943 Iron Iii Chloride Charge Some of the metals form very common ions which have latin names that are in. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii) chloride has a charge of #3^+#. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged.. Iron Iii Chloride Charge.
From www.researchgate.net
What is the chemical difference between these iron III chloride Iron Iii Chloride Charge 3.1 introduction to the octet rule. Some of the metals form very common ions which have latin names that are in. Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. therefore, the iron cation in iron(ii) chloride has a charge of #2^+#, and the charge on the iron cation in iron(iii). Iron Iii Chloride Charge.
From exombjkja.blob.core.windows.net
Iron 3 Chloride Ligands at Linda Chase blog Iron Iii Chloride Charge For example, iron( ii ) has a. 3.1 introduction to the octet rule. so fe +2 is iron(ii) and fe +3 is iron(iii). Some of the metals form very common ions which have latin names that are in. In the simpler, more modern approach, called the stock system, an ion’s positive charge. This is because the number of electrons. Iron Iii Chloride Charge.
From www.youtube.com
Iron(iii) Chloride Preparation YouTube Iron Iii Chloride Charge Some of the metals form very common ions which have latin names that are in. there are two ways to make this distinction. so fe +2 is iron(ii) and fe +3 is iron(iii). roman numeral notation indicates charge of ion when element commonly forms more than one ion. In the simpler, more modern approach, called the stock. Iron Iii Chloride Charge.
From www.chegg.com
Solved (4pts) For the reaction of iron(III) chloride and Iron Iii Chloride Charge In the simpler, more modern approach, called the stock system, an ion’s positive charge. so fe +2 is iron(ii) and fe +3 is iron(iii). iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. Some of the metals form very common ions which have latin names that are in. Up until now we. Iron Iii Chloride Charge.
From www.researchgate.net
What is the chemical difference between these iron III chloride Iron Iii Chloride Charge In the simpler, more modern approach, called the stock system, an ion’s positive charge. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry. Some of the metals form very common ions which have. Iron Iii Chloride Charge.
From www.abmstore.my
Iron 3 Chloride Anhydrous HmbG 500g AbmStore.my Iron Iii Chloride Charge Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. In the simpler, more modern approach, called the stock system, an ion’s positive charge. 3.1 introduction to the octet rule. For example, iron( ii ) has a. iron(iii) chloride, chemically represented as fecl3, holds a pivotal position in the realm of chemistry.. Iron Iii Chloride Charge.