Mace-4 Primary Endpoint . Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. What is an efficacy endpoint? Ahre was defined as > 175 bpm. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. • a measure designed to reflect the intended effects of a drug. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Guidance for industry multiple endpoints in.
from www.slideserve.com
Ahre was defined as > 175 bpm. What is an efficacy endpoint? • a measure designed to reflect the intended effects of a drug. Guidance for industry multiple endpoints in. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”.
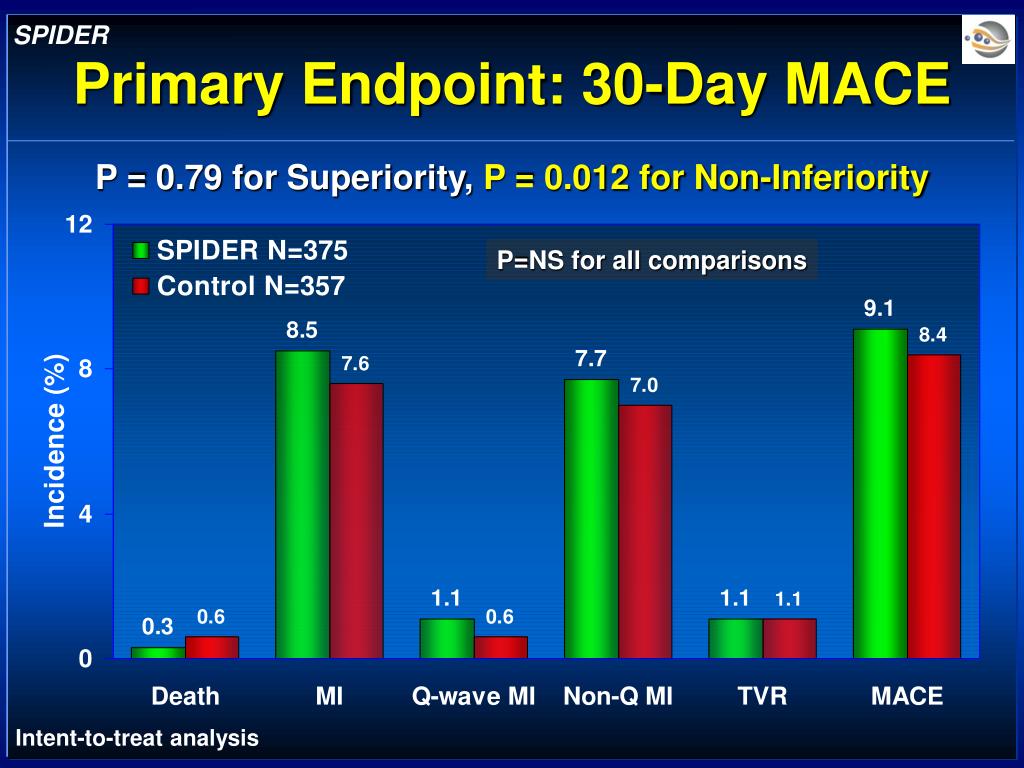
PPT SPIDER S aphenous Vein Graft P rotection I n a D istal E mbolic
Mace-4 Primary Endpoint The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. • a measure designed to reflect the intended effects of a drug. Guidance for industry multiple endpoints in. Ahre was defined as > 175 bpm. What is an efficacy endpoint? We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h.
From www.slideserve.com
PPT Multiple Primary Endpoints in Clinical Trials PowerPoint Mace-4 Primary Endpoint The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Guidance for industry multiple endpoints in. • a measure designed to reflect the intended effects of a drug. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. What is an efficacy. Mace-4 Primary Endpoint.
From www.researchgate.net
Rate of the primary and secondary endpoints at the 3year followup Mace-4 Primary Endpoint Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. Ahre was defined as > 175 bpm. • a measure designed to reflect the intended effects of a drug. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥. Mace-4 Primary Endpoint.
From openheart.bmj.com
Randomised comparison of provisional side branch stenting versus a two Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. What is an efficacy endpoint? Ahre was defined as > 175. Mace-4 Primary Endpoint.
From www.researchgate.net
Comparison of MACE (4 years) in two groups. Download Scientific Diagram Mace-4 Primary Endpoint Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. • a measure designed to reflect the intended effects of a drug. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. The primary outcome was a composite endpoint of mace. Mace-4 Primary Endpoint.
From slideplayer.com
Angioplasty Balloonassociated Coronary Debris and the EZ FilterWire Mace-4 Primary Endpoint Ahre was defined as > 175 bpm. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. • a measure designed to reflect the intended effects of a drug. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. Guidance for. Mace-4 Primary Endpoint.
From slideplayer.com
CV Risk Management in T2DM What Did We Learn from ADA 2016? ppt download Mace-4 Primary Endpoint We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. • a measure designed to reflect the intended effects of a drug. Ahre was defined as > 175 bpm. Guidance for industry multiple endpoints in. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and. Mace-4 Primary Endpoint.
From www.keyamedical.com
TARGET Randomized OnSite CT FFR Trial Results KeyaMedical Mace-4 Primary Endpoint • a measure designed to reflect the intended effects of a drug. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. Ahre was defined as > 175 bpm. What is an efficacy endpoint? The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥. Mace-4 Primary Endpoint.
From www.invivochem.com
Semaglutide agonist of glucagonlike peptide1 (GLP1) receptor CAS Mace-4 Primary Endpoint Ahre was defined as > 175 bpm. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. What is an efficacy endpoint? Guidance for industry multiple endpoints in. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. • a measure designed. Mace-4 Primary Endpoint.
From slideplayer.com
Study Design AMI Mace-4 Primary Endpoint Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. Guidance for industry multiple endpoints in. Ahre was defined as > 175 bpm. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. • a measure designed to reflect the intended. Mace-4 Primary Endpoint.
From www.researchgate.net
(PDF) Retrospective Study of Appropriate Primary Prevention in Mace-4 Primary Endpoint • a measure designed to reflect the intended effects of a drug. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. What is an efficacy endpoint? Ahre was defined as > 175 bpm. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥. Mace-4 Primary Endpoint.
From www.campus.sanofi
ODYSSEY Mace-4 Primary Endpoint Ahre was defined as > 175 bpm. Guidance for industry multiple endpoints in. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. What is an efficacy endpoint? Cardiovascular outcomes trials (cvots). Mace-4 Primary Endpoint.
From slideplayer.com
Risk Stratification in CAD and PAD ppt download Mace-4 Primary Endpoint What is an efficacy endpoint? Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. Guidance for industry multiple endpoints in. • a measure designed to reflect the intended effects of a drug. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥. Mace-4 Primary Endpoint.
From slideplayer.com
Potential conflicts of interest ppt download Mace-4 Primary Endpoint We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. • a measure designed to reflect the intended effects of a drug. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. Guidance for industry multiple endpoints in. Ahre was defined. Mace-4 Primary Endpoint.
From slideplayer.com
Randomized Comparison of a CrossBoss First vs ppt download Mace-4 Primary Endpoint We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. What is an efficacy endpoint? • a measure designed to reflect the intended effects of a drug. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Guidance for industry multiple endpoints. Mace-4 Primary Endpoint.
From www.pngkey.com
Download Cto Systems Primary Safety Endpoint 30day Mace Massey Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. What is an efficacy endpoint? The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2. Mace-4 Primary Endpoint.
From slidetodoc.com
Atlantic Cardiovascular Patient Research Team CPORT E Mace-4 Primary Endpoint The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative.. Mace-4 Primary Endpoint.
From www.researchgate.net
(PDF) Statistical power for MACE and individual secondary endpoints in Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. • a measure designed to reflect the intended effects of a drug. Ahre was defined as > 175 bpm. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major. Mace-4 Primary Endpoint.
From slidetodoc.com
Effect of BET Protein Inhibition With Apabetalone on Mace-4 Primary Endpoint • a measure designed to reflect the intended effects of a drug. Ahre was defined as > 175 bpm. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. The primary. Mace-4 Primary Endpoint.
From www.patientcareonline.com
Bempedoic Acid Meets Primary Endpoint in Key Cardiovascular Mace-4 Primary Endpoint Ahre was defined as > 175 bpm. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. Guidance for industry multiple endpoints in. • a measure designed to reflect the intended. Mace-4 Primary Endpoint.
From slideplayer.com
Randomized Comparison of a CrossBoss First vs ppt download Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. • a measure designed to reflect the intended effects of a drug. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24. Mace-4 Primary Endpoint.
From slideplayer.com
SORTOUT III A Prospective Randomized Comparison of Zotarolimus Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. What is an efficacy endpoint? • a measure designed to reflect the intended effects of a drug. Ahre was defined as > 175 bpm. We included studies that identified mace composite endpoints as. Mace-4 Primary Endpoint.
From slideplayer.com
The Elevated Role of GLP1 RAs in Diabetes Management Which Patients Mace-4 Primary Endpoint Ahre was defined as > 175 bpm. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. Guidance for industry multiple endpoints in. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. • a measure designed. Mace-4 Primary Endpoint.
From www.scribd.com
Mace 4 9math PDF Pressure Variable (Mathematics) Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Ahre was defined as > 175 bpm. • a measure designed to reflect the intended effects of a drug. What is an efficacy endpoint? Cardiovascular outcomes trials (cvots) with novel drugs to treat. Mace-4 Primary Endpoint.
From www.researchgate.net
Freedom from primary endpoint at 376 ± 18.1 days of followup (MACE Mace-4 Primary Endpoint • a measure designed to reflect the intended effects of a drug. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. Cardiovascular outcomes trials (cvots) with novel drugs to treat type. Mace-4 Primary Endpoint.
From www.researchgate.net
Definition of study endpoints, MACE, and adjusted confounders in the Mace-4 Primary Endpoint The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. • a measure designed to reflect the intended effects of a drug. Ahre was defined as >. Mace-4 Primary Endpoint.
From slideplayer.com
On behalf of Multistrategy ppt download Mace-4 Primary Endpoint The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. Ahre was defined as > 175 bpm. We included studies that identified mace composite endpoints as the. Mace-4 Primary Endpoint.
From www.slideserve.com
PPT SPIDER S aphenous Vein Graft P rotection I n a D istal E mbolic Mace-4 Primary Endpoint The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. What is an efficacy endpoint? • a measure designed to reflect the intended effects of a drug. Cardiovascular outcomes trials (cvots) with. Mace-4 Primary Endpoint.
From www.slideserve.com
PPT What are Important Endpoints in Anaesthesia Research? PowerPoint Mace-4 Primary Endpoint Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. Ahre was defined as > 175 bpm. • a measure designed to reflect the intended effects of a drug. The primary. Mace-4 Primary Endpoint.
From www.nature.com
Statistical power for MACE and individual secondary endpoints in Mace-4 Primary Endpoint • a measure designed to reflect the intended effects of a drug. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. Ahre was defined as > 175 bpm. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular. Mace-4 Primary Endpoint.
From www.researchgate.net
Freedom from primary endpoint at 376 ± 18.1 days of followup (MACE Mace-4 Primary Endpoint The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. What is an efficacy endpoint? • a measure designed to reflect the intended effects of a drug. Guidance for industry multiple endpoints. Mace-4 Primary Endpoint.
From www.slideserve.com
PPT Gregg W. Stone MD For the AMIHOT II Investigators PowerPoint Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. What is an efficacy endpoint? Ahre was defined as > 175 bpm. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. • a measure designed to reflect the intended effects of a drug. Cardiovascular outcomes trials (cvots) with novel drugs to treat. Mace-4 Primary Endpoint.
From www.researchgate.net
Overview of the MACE framework. Download Scientific Diagram Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. • a measure designed to reflect the intended effects of a drug. What is an efficacy endpoint? The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24. Mace-4 Primary Endpoint.
From www.researchgate.net
Risk factors of MACE and its components after RACABG. CV death Mace-4 Primary Endpoint Ahre was defined as > 175 bpm. Guidance for industry multiple endpoints in. The primary outcome was a composite endpoint of mace after ahre ≥ 5 min, ≥ 6 h, and ≥ 24 h. What is an efficacy endpoint? We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. Cardiovascular outcomes trials (cvots). Mace-4 Primary Endpoint.
From slidetodoc.com
DECLARE TIMI 58 Stephen D Wiviott MD for Mace-4 Primary Endpoint Guidance for industry multiple endpoints in. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. • a measure designed to reflect the intended effects of a drug. Ahre was defined as > 175 bpm. The primary outcome was a composite endpoint of mace after ahre ≥ 5. Mace-4 Primary Endpoint.
From www.researchgate.net
Study endpoints and definitions Study endpoints Primary endpoint Mace-4 Primary Endpoint What is an efficacy endpoint? Guidance for industry multiple endpoints in. Cardiovascular outcomes trials (cvots) with novel drugs to treat type 2 diabetes have uniformly chosen the composite “major adverse cardiovascular events (mace)”. We included studies that identified mace composite endpoints as the primary or secondary study outcome, used administrative. The primary outcome was a composite endpoint of mace after. Mace-4 Primary Endpoint.