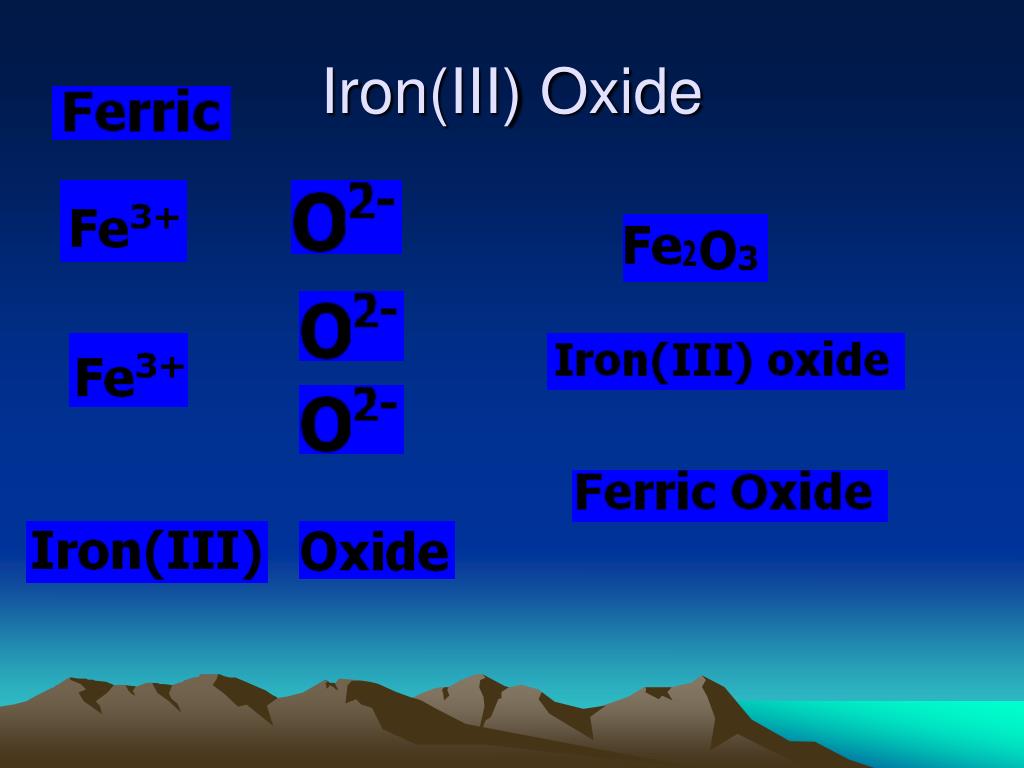
Iron Iii Oxide Positive And Negative Ion . To balance the positive and. The oxide has a 2− charge as an ion. Ionic compounds have positive ions and negative ions. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Iron can form two possible ions, but the ion with a 3+ charge is specified here. To balance the positive and negative charges, we look to the least. The oxidation state of fe 2 o 3 is +3. Compounds formed from positive and negative ions are ionic compounds. The oxygen atom has a 2− charge as an ion. Individual atoms can gain or lose electrons. Ionic formulas balance the total positive and negative charges. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. This is two positive charges and one negative charge;
from www.slideserve.com
The oxygen atom has a 2− charge as an ion. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. Ionic formulas balance the total positive and negative charges. The oxidation state of fe 2 o 3 is +3. Individual atoms can gain or lose electrons. Iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxide has a 2− charge as an ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Compounds formed from positive and negative ions are ionic compounds. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms.
PPT Naming Type (I) and (II) Compounds PowerPoint Presentation, free
Iron Iii Oxide Positive And Negative Ion To balance the positive and negative charges, we look to the least. The oxygen atom has a 2− charge as an ion. The oxide has a 2− charge as an ion. To balance the positive and. Compounds formed from positive and negative ions are ionic compounds. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Ionic formulas balance the total positive and negative charges. The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. To balance the positive and negative charges, we look to the least. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Individual atoms can gain or lose electrons. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. This is two positive charges and one negative charge; Ionic compounds have positive ions and negative ions.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron Iii Oxide Positive And Negative Ion Ionic compounds have positive ions and negative ions. The oxide has a 2− charge as an ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Iron(iii), fe 3+ working out a formula the formula for an. Iron Iii Oxide Positive And Negative Ion.
From www.coursehero.com
[Solved] Iron (III) oxide reacts with carbon monoxide to produce Iron Iii Oxide Positive And Negative Ion Ionic compounds have positive ions and negative ions. Individual atoms can gain or lose electrons. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Compounds formed from positive and negative ions are ionic compounds. Ionic formulas balance the total positive and negative charges. Iron can form two possible ions, but the ion with. Iron Iii Oxide Positive And Negative Ion.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses Iron Iii Oxide Positive And Negative Ion This is two positive charges and one negative charge; Compounds formed from positive and negative ions are ionic compounds. Ionic compounds have positive ions and negative ions. Iron can form two possible ions, but the ion with a 3+ charge is specified here. To balance the positive and negative charges, we look to the least. Fe 2 o 3 is. Iron Iii Oxide Positive And Negative Ion.
From testbook.com
Oxide Learn Structure, Preparation, Properties & Uses Iron Iii Oxide Positive And Negative Ion The oxygen atom has a 2− charge as an ion. The oxidation state of fe 2 o 3 is +3. The oxide has a 2− charge as an ion. To balance the positive and negative charges, we look to the least. Iron can form two possible ions, but the ion with a 3+ charge is specified here. To balance the. Iron Iii Oxide Positive And Negative Ion.
From slideplayer.com
Ionic Compounds Naming ppt download Iron Iii Oxide Positive And Negative Ion The oxide has a 2− charge as an ion. This is two positive charges and one negative charge; Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. To balance the positive and negative charges, we look to the least. Ionic formulas balance the total positive. Iron Iii Oxide Positive And Negative Ion.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron Iii Oxide Positive And Negative Ion To balance the positive and. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxygen atom has a 2− charge as an ion. Iron(iii), fe 3+ working out a formula the. Iron Iii Oxide Positive And Negative Ion.
From stock.adobe.com
Iron Iii Oxide Properties and Chemical Compound Structure Stock Vector Iron Iii Oxide Positive And Negative Ion The oxidation state of fe 2 o 3 is +3. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. To balance the positive and negative charges, we look to the least. To. Iron Iii Oxide Positive And Negative Ion.
From slidetodoc.com
Writing and Naming Chemical Formulas for Ionic Compounds Iron Iii Oxide Positive And Negative Ion The oxide has a 2− charge as an ion. Ionic formulas balance the total positive and negative charges. Individual atoms can gain or lose electrons. To balance the positive and negative charges, we look to the least. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Fe 2 o 3 is the chemical. Iron Iii Oxide Positive And Negative Ion.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Iii Oxide Positive And Negative Ion Ionic formulas balance the total positive and negative charges. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. The oxygen atom has a 2− charge as an ion. This is two positive charges and one negative charge; Iron(iii), fe 3+ working out a formula the formula for an. Iron Iii Oxide Positive And Negative Ion.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Iron Iii Oxide Positive And Negative Ion To balance the positive and negative charges, we look to the least. The oxidation state of fe 2 o 3 is +3. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. To. Iron Iii Oxide Positive And Negative Ion.
From www.chegg.com
Solved Positive ion Negative ion Formula Name Copper( ) Iron Iii Oxide Positive And Negative Ion This is two positive charges and one negative charge; Ionic formulas balance the total positive and negative charges. The oxide has a 2− charge as an ion. To balance the positive and. The oxidation state of fe 2 o 3 is +3. To balance the positive and negative charges, we look to the least. Iron can form two possible ions,. Iron Iii Oxide Positive And Negative Ion.
From www.slideserve.com
PPT Ionic Compounds Naming PowerPoint Presentation, free download Iron Iii Oxide Positive And Negative Ion To balance the positive and negative charges, we look to the least. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. The oxygen atom has a 2− charge as an ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Individual. Iron Iii Oxide Positive And Negative Ion.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron Iii Oxide Positive And Negative Ion To balance the positive and. Iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. This is two positive charges and one negative. Iron Iii Oxide Positive And Negative Ion.
From www.youtube.com
What is the formula for iron III oxide? YouTube Iron Iii Oxide Positive And Negative Ion The oxidation state of fe 2 o 3 is +3. Ionic compounds have positive ions and negative ions. The oxide has a 2− charge as an ion. To balance the positive and negative charges, we look to the least. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms.. Iron Iii Oxide Positive And Negative Ion.
From www.slideserve.com
PPT Naming Type (I) and (II) Compounds PowerPoint Presentation, free Iron Iii Oxide Positive And Negative Ion Compounds formed from positive and negative ions are ionic compounds. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Individual atoms can gain or lose electrons. Ionic formulas balance the total positive and negative charges. To balance the positive and negative charges, we look to the least. Iron can form two possible ions,. Iron Iii Oxide Positive And Negative Ion.
From www.youtube.com
Lewis Structure of Fe2O3, Iron (III) Oxide YouTube Iron Iii Oxide Positive And Negative Ion The oxidation state of fe 2 o 3 is +3. Individual atoms can gain or lose electrons. To balance the positive and. The oxygen atom has a 2− charge as an ion. To balance the positive and negative charges, we look to the least. Iron can form two possible ions, but the ion with a 3+ charge is specified here.. Iron Iii Oxide Positive And Negative Ion.
From www.slideserve.com
PPT Unit Chemical Reactions PowerPoint Presentation, free download Iron Iii Oxide Positive And Negative Ion The oxygen atom has a 2− charge as an ion. Individual atoms can gain or lose electrons. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. Compounds formed from positive and negative ions are ionic compounds. Fe 2 o 3 is the chemical formula of. Iron Iii Oxide Positive And Negative Ion.
From www.slideserve.com
PPT Nomenclature PowerPoint Presentation, free download Iron Iii Oxide Positive And Negative Ion Ionic formulas balance the total positive and negative charges. Ionic compounds have positive ions and negative ions. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. To balance the positive and. Individual atoms can gain or lose electrons. The oxygen atom has a 2− charge. Iron Iii Oxide Positive And Negative Ion.
From examples.yourdictionary.com
Ion Examples With Positive & Negative Charges Iron Iii Oxide Positive And Negative Ion Individual atoms can gain or lose electrons. The oxide has a 2− charge as an ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxygen atom has a 2− charge as an ion. Compounds formed from positive and negative ions are ionic compounds. The oxidation state of fe 2 o 3. Iron Iii Oxide Positive And Negative Ion.
From www.youtube.com
Is Fe2O3, Iron (III) oxide. Ionic or Covalent? YouTube Iron Iii Oxide Positive And Negative Ion To balance the positive and. Ionic formulas balance the total positive and negative charges. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Individual atoms can gain or lose. Iron Iii Oxide Positive And Negative Ion.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Iron Iii Oxide Positive And Negative Ion Iron can form two possible ions, but the ion with a 3+ charge is specified here. This is two positive charges and one negative charge; The oxide has a 2− charge as an ion. Compounds formed from positive and negative ions are ionic compounds. Ionic compounds have positive ions and negative ions. Iron can form two possible ions, but the. Iron Iii Oxide Positive And Negative Ion.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Iron Iii Oxide Positive And Negative Ion This is two positive charges and one negative charge; Individual atoms can gain or lose electrons. Iron can form two possible ions, but the ion with a 3+ charge is specified here. To balance the positive and. The oxide has a 2− charge as an ion. Compounds formed from positive and negative ions are ionic compounds. Iron can form two. Iron Iii Oxide Positive And Negative Ion.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube Iron Iii Oxide Positive And Negative Ion Iron can form two possible ions, but the ion with a 3+ charge is specified here. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Ionic formulas balance the total positive and negative charges. The oxide has a 2− charge as an ion. The oxidation state of fe. Iron Iii Oxide Positive And Negative Ion.
From en.wikipedia.org
Iron(III) oxide Wikipedia Iron Iii Oxide Positive And Negative Ion This is two positive charges and one negative charge; Compounds formed from positive and negative ions are ionic compounds. Ionic compounds have positive ions and negative ions. The oxide has a 2− charge as an ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxidation state of fe 2 o 3. Iron Iii Oxide Positive And Negative Ion.
From www.youtube.com
Naming Ionic compounds Iron (III) Oxide YouTube Iron Iii Oxide Positive And Negative Ion Ionic formulas balance the total positive and negative charges. The oxygen atom has a 2− charge as an ion. The oxidation state of fe 2 o 3 is +3. Compounds formed from positive and negative ions are ionic compounds. The oxide has a 2− charge as an ion. Ionic compounds have positive ions and negative ions. Iron can form two. Iron Iii Oxide Positive And Negative Ion.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector Iron Iii Oxide Positive And Negative Ion To balance the positive and. Compounds formed from positive and negative ions are ionic compounds. Ionic compounds have positive ions and negative ions. The oxide has a 2− charge as an ion. The oxygen atom has a 2− charge as an ion. This is two positive charges and one negative charge; Iron(iii), fe 3+ working out a formula the formula. Iron Iii Oxide Positive And Negative Ion.
From slideplayer.com
Unit 2 Chapter 5 Ionic Compound Names ppt download Iron Iii Oxide Positive And Negative Ion The oxidation state of fe 2 o 3 is +3. To balance the positive and. Iron can form two possible ions, but the ion with a 3+ charge is specified here. This is two positive charges and one negative charge; The oxygen atom has a 2− charge as an ion. Iron(iii), fe 3+ working out a formula the formula for. Iron Iii Oxide Positive And Negative Ion.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron Iii Oxide Positive And Negative Ion The oxide has a 2− charge as an ion. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Iron can form two possible ions,. Iron Iii Oxide Positive And Negative Ion.
From www.chegg.com
Solved 2. Formulas of ionic compounds Name Positive Ion Iron Iii Oxide Positive And Negative Ion Ionic compounds have positive ions and negative ions. To balance the positive and negative charges, we look to the least. Individual atoms can gain or lose electrons. Ionic formulas balance the total positive and negative charges. The oxygen atom has a 2− charge as an ion. The oxidation state of fe 2 o 3 is +3. Iron(iii), fe 3+ working. Iron Iii Oxide Positive And Negative Ion.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron Iii Oxide Positive And Negative Ion The oxide has a 2− charge as an ion. Compounds formed from positive and negative ions are ionic compounds. This is two positive charges and one negative charge; To balance the positive and negative charges, we look to the least. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron. Iron Iii Oxide Positive And Negative Ion.
From slideplayer.com
Ionic Compounds Naming ppt download Iron Iii Oxide Positive And Negative Ion To balance the positive and. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. Ionic compounds have positive ions and negative ions. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. This is. Iron Iii Oxide Positive And Negative Ion.
From www.slideserve.com
PPT Combustion analysis PowerPoint Presentation, free download ID Iron Iii Oxide Positive And Negative Ion The oxygen atom has a 2− charge as an ion. The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of. Iron Iii Oxide Positive And Negative Ion.
From meaningkosh.com
Iron 3 Oxide MeaningKosh Iron Iii Oxide Positive And Negative Ion To balance the positive and. Ionic formulas balance the total positive and negative charges. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Ionic compounds have positive ions and negative ions. Compounds formed from positive and negative ions are ionic compounds. The oxygen atom has a 2− charge as an ion. Individual atoms. Iron Iii Oxide Positive And Negative Ion.
From www.slideserve.com
PPT Ionic Bonding in Review PowerPoint Presentation, free download Iron Iii Oxide Positive And Negative Ion Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Iron(iii), fe 3+ working out a formula the formula for an ionic compound must contain the same number of positive and negative charges close. To balance the positive and. Compounds formed from positive and negative ions are ionic compounds.. Iron Iii Oxide Positive And Negative Ion.
From www.chegg.com
Solved 2. Formulas of ionic compounds Name Positive Ion Iron Iii Oxide Positive And Negative Ion Ionic compounds have positive ions and negative ions. The oxide has a 2− charge as an ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. To balance the positive and. Fe 2 o 3 is the chemical formula of iron (iii) oxide which has three oxygen atoms, and two iron atoms. Iron(iii),. Iron Iii Oxide Positive And Negative Ion.