Evaporation Deposition Melting . Carbon dioxide is an example of a material that easily undergoes. In evaporation, a liquid turns into a gas; The opposite process is condensation. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of a material that easily undergoes. Deposition is the change of state from a gas to a solid. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Define melting, freezing, vaporization, condensation, sublimation, and deposition. A substance melts or freezes at a temperature.
from www.numerade.com
A substance melts or freezes at a temperature. Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of a material that easily undergoes. The opposite process is condensation. In evaporation, a liquid turns into a gas; Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Deposition is the change of state from a gas to a solid. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Carbon dioxide is an example of a material that easily undergoes.
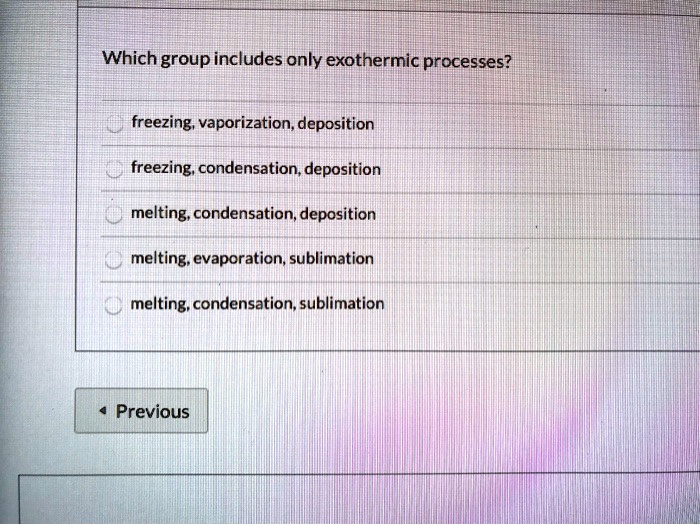
SOLVED Which group includes only exothermic processes? freezing
Evaporation Deposition Melting Carbon dioxide is an example of a material that easily undergoes. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. In evaporation, a liquid turns into a gas; Define melting, freezing, vaporization, condensation, sublimation, and deposition. A substance melts or freezes at a temperature. The opposite process is condensation. Carbon dioxide is an example of a material that easily undergoes. Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of a material that easily undergoes. Deposition is the change of state from a gas to a solid. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation.
From www.numerade.com
SOLVED Which group includes only exothermic processes? freezing Evaporation Deposition Melting Define melting, freezing, vaporization, condensation, sublimation, and deposition. Deposition is the change of state from a gas to a solid. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. In evaporation, a liquid turns into a gas; Carbon dioxide is. Evaporation Deposition Melting.
From www.youtube.com
Change in States of matter Freezing Condensation Melting Evaporation Deposition Melting A substance melts or freezes at a temperature. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. In evaporation, a liquid turns into a gas; Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of a material that easily undergoes. Carbon dioxide is an example of. Evaporation Deposition Melting.
From data.allenai.org
changes of state (lesson 0771) TQA explorer Evaporation Deposition Melting Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Carbon dioxide is an example of a material that easily undergoes. A substance melts or freezes at a temperature. In evaporation, a liquid turns into a gas; Deposition is the change. Evaporation Deposition Melting.
From slideplayer.com
Unit 1 Introduction to Chemistry ppt download Evaporation Deposition Melting Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Deposition is the change of state from a gas to a solid. Deposition is the change of state from a gas to a solid. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. A substance melts or freezes at a. Evaporation Deposition Melting.
From www.shmoop.com
Chemistry Phase Changes Shmoop Chemistry Evaporation Deposition Melting Deposition is the change of state from a gas to a solid. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Deposition is the change of state from a gas to a solid. The opposite process is condensation. Carbon dioxide is an example of a material that easily undergoes. Define melting, freezing, vaporization, condensation,. Evaporation Deposition Melting.
From slideplayer.com
Matter Because it matters. ppt download Evaporation Deposition Melting A substance melts or freezes at a temperature. Deposition is the change of state from a gas to a solid. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Carbon dioxide is an example of a material that easily undergoes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. In evaporation, a liquid turns into. Evaporation Deposition Melting.
From www.expii.com
Melting — Definition & Overview Expii Evaporation Deposition Melting Define melting, freezing, vaporization, condensation, sublimation, and deposition. Carbon dioxide is an example of a material that easily undergoes. A substance melts or freezes at a temperature. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Deposition is the change. Evaporation Deposition Melting.
From vdocuments.mx
Phase changes Melting Freezing Vaporization (or evaporation Evaporation Deposition Melting Carbon dioxide is an example of a material that easily undergoes. Deposition is the change of state from a gas to a solid. The opposite process is condensation. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. In evaporation, a liquid turns into a gas; Deposition is the change of state from a gas. Evaporation Deposition Melting.
From www.coursehero.com
[Solved] Sublimation Evaporation Deposition Condensation Melting Evaporation Deposition Melting Carbon dioxide is an example of a material that easily undergoes. The opposite process is condensation. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Deposition is the change of state from a gas to a solid. A substance melts or freezes at a temperature. In evaporation, a liquid turns into a gas; Energy changes. Evaporation Deposition Melting.
From data.allenai.org
changes of state (lesson 0771) TQA explorer Evaporation Deposition Melting Carbon dioxide is an example of a material that easily undergoes. Deposition is the change of state from a gas to a solid. A substance melts or freezes at a temperature. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Carbon dioxide is an example of. Evaporation Deposition Melting.
From www.numerade.com
SOLVED Question 35 (2 points) Which pair of processes are both Evaporation Deposition Melting In evaporation, a liquid turns into a gas; A substance melts or freezes at a temperature. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Deposition is the change of state from a gas to a solid. Deposition is the change of state from a gas to a solid. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation,. Evaporation Deposition Melting.
From www.semanticscholar.org
Figure 21 from Mathematical modeling of the melt pool during a Evaporation Deposition Melting A substance melts or freezes at a temperature. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Carbon dioxide is an example of a material that easily undergoes. Deposition is the change of state from a gas to a solid. Deposition is the change of state from a gas to a solid. Define melting,. Evaporation Deposition Melting.
From mavink.com
Melting Phase Diagram Evaporation Deposition Melting Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. A substance melts or freezes at a temperature. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. The opposite process is condensation. Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of. Evaporation Deposition Melting.
From app.emaze.com
on emaze Evaporation Deposition Melting Carbon dioxide is an example of a material that easily undergoes. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Carbon dioxide is an example of a material that easily undergoes. In evaporation, a liquid turns into a gas; Define melting, freezing, vaporization, condensation, sublimation, and deposition. A substance melts or freezes at a temperature.. Evaporation Deposition Melting.
From brainly.ph
choices (melting,deposition,condensation, evaporation,freezing Evaporation Deposition Melting In evaporation, a liquid turns into a gas; A substance melts or freezes at a temperature. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. The opposite process is condensation. Carbon dioxide. Evaporation Deposition Melting.
From www.scribd.com
Freezing, Melting, and Evaporation PDF Melting Point Evaporation Evaporation Deposition Melting A substance melts or freezes at a temperature. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Deposition is the change of state from a gas to a solid. The opposite process is condensation. In evaporation, a liquid turns into a gas; Deposition is the change of state from a gas to a solid.. Evaporation Deposition Melting.
From www.pinterest.com
States of Matter Unit Melting, Freezing, Evaporation, Condensation Evaporation Deposition Melting Define melting, freezing, vaporization, condensation, sublimation, and deposition. Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of a material that easily undergoes. In evaporation, a liquid turns into a gas; Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. The opposite process is condensation. Energy changes. Evaporation Deposition Melting.
From www.youtube.com
Change of State Evaporation Condensation Melting Freezing Evaporation Deposition Melting Deposition is the change of state from a gas to a solid. The opposite process is condensation. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. In evaporation, a liquid turns into. Evaporation Deposition Melting.
From stock.adobe.com
Vector diagram with changing states of matter, three states of matter Evaporation Deposition Melting Deposition is the change of state from a gas to a solid. A substance melts or freezes at a temperature. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. The opposite process is condensation. Carbon dioxide is an example of a material that easily undergoes. In evaporation, a liquid turns into a gas; Define melting,. Evaporation Deposition Melting.
From stock.adobe.com
Physical states of matter.Solid, liquid and gas.Melting, freezing Evaporation Deposition Melting Deposition is the change of state from a gas to a solid. A substance melts or freezes at a temperature. Carbon dioxide is an example of a material that easily undergoes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. The opposite process is condensation. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Energy changes. Evaporation Deposition Melting.
From slideplayer.com
Changes of State Melting, Freezing, Vaporization, Evaporation Evaporation Deposition Melting The opposite process is condensation. In evaporation, a liquid turns into a gas; Carbon dioxide is an example of a material that easily undergoes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Deposition is the change of state from a gas to a solid. Deposition. Evaporation Deposition Melting.
From education-portal.com
Phase Change Evaporation, Condensation, Freezing, Melting, Sublimation Evaporation Deposition Melting A substance melts or freezes at a temperature. In evaporation, a liquid turns into a gas; The opposite process is condensation. Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of a material that easily undergoes. Carbon dioxide is an example of a material that easily undergoes. Energy changes in sublimation/deposition are. Evaporation Deposition Melting.
From www.pinterest.co.kr
States of Water infographic diagram including gas liquid and solid also Evaporation Deposition Melting Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Carbon dioxide is an example of a material that easily undergoes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Deposition is the change of state from a gas to a solid. The opposite process is condensation. A substance melts or freezes at a temperature. Deposition is. Evaporation Deposition Melting.
From www.pinterest.com
Phase Change Evaporation, Condensation, Freezing, Melting, Sublimation Evaporation Deposition Melting Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Carbon dioxide is an example of a material that easily undergoes. Deposition is the change of state from a gas to a solid. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Carbon dioxide is an example of a material. Evaporation Deposition Melting.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition Evaporation Deposition Melting The opposite process is condensation. Deposition is the change of state from a gas to a solid. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Carbon dioxide is an example of a material that easily undergoes. A substance melts. Evaporation Deposition Melting.
From www.numerade.com
SOLVED Question 3 Write Endothermic or Exothermic to these processes Evaporation Deposition Melting In evaporation, a liquid turns into a gas; Define melting, freezing, vaporization, condensation, sublimation, and deposition. A substance melts or freezes at a temperature. Carbon dioxide is an example of a material that easily undergoes. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Carbon dioxide is an example of a material that easily undergoes.. Evaporation Deposition Melting.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Evaporation Deposition Melting Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of a material that easily undergoes. Carbon dioxide is an example of a material that easily undergoes. A substance melts or freezes at a temperature. Define melting, freezing, vaporization, condensation, sublimation, and deposition. The opposite process is condensation. In evaporation, a liquid turns. Evaporation Deposition Melting.
From www.pinterest.com
Phase Change Evaporation, Condensation, Freezing, Melting, Sublimation Evaporation Deposition Melting Carbon dioxide is an example of a material that easily undergoes. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. In evaporation, a liquid turns into a gas; Carbon dioxide is an example of a material that easily undergoes. A. Evaporation Deposition Melting.
From ecampusontario.pressbooks.pub
13.3 Phase Transitions Enhanced Introductory College Chemistry Evaporation Deposition Melting Carbon dioxide is an example of a material that easily undergoes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. The opposite process is condensation. In evaporation, a liquid turns into a gas; Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. A substance melts or freezes at a temperature. Deposition is the change of state. Evaporation Deposition Melting.
From www.youtube.com
Phase transitionsStates of matterMeltingvaporizationDeposition Evaporation Deposition Melting Carbon dioxide is an example of a material that easily undergoes. The opposite process is condensation. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Deposition is the change of state from a gas to a solid. Deposition is the change of state from a gas to. Evaporation Deposition Melting.
From www.numerade.com
Determine whether heating or cooling takes place during each process Evaporation Deposition Melting In evaporation, a liquid turns into a gas; The opposite process is condensation. A substance melts or freezes at a temperature. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Deposition is the change of state from a gas to a solid. Processes involved in changes. Evaporation Deposition Melting.
From www.slideshare.net
(Science) Matter Evaporation Deposition Melting Define melting, freezing, vaporization, condensation, sublimation, and deposition. A substance melts or freezes at a temperature. Deposition is the change of state from a gas to a solid. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Deposition is the change of state from a gas to a solid. The opposite process is condensation. Carbon. Evaporation Deposition Melting.
From www.youtube.com
Changing states of matter 🔁 Melting, Freezing, Evaporation Evaporation Deposition Melting Deposition is the change of state from a gas to a solid. In evaporation, a liquid turns into a gas; The opposite process is condensation. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Carbon dioxide is an example of a material that easily undergoes. Carbon dioxide is an example of a material that. Evaporation Deposition Melting.
From stock.adobe.com
Change in state with gas, liquid and solid water outline diagram Evaporation Deposition Melting In evaporation, a liquid turns into a gas; The opposite process is condensation. Deposition is the change of state from a gas to a solid. Carbon dioxide is an example of a material that easily undergoes. Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation. Carbon dioxide is an example of a material that easily. Evaporation Deposition Melting.
From askfilo.com
Deposition, evaporation, melting point, condensation, sublimation and boi.. Evaporation Deposition Melting Deposition is the change of state from a gas to a solid. Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas. Carbon dioxide is an example of a material that easily undergoes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. In evaporation, a liquid turns into a gas; Carbon dioxide is an example of. Evaporation Deposition Melting.