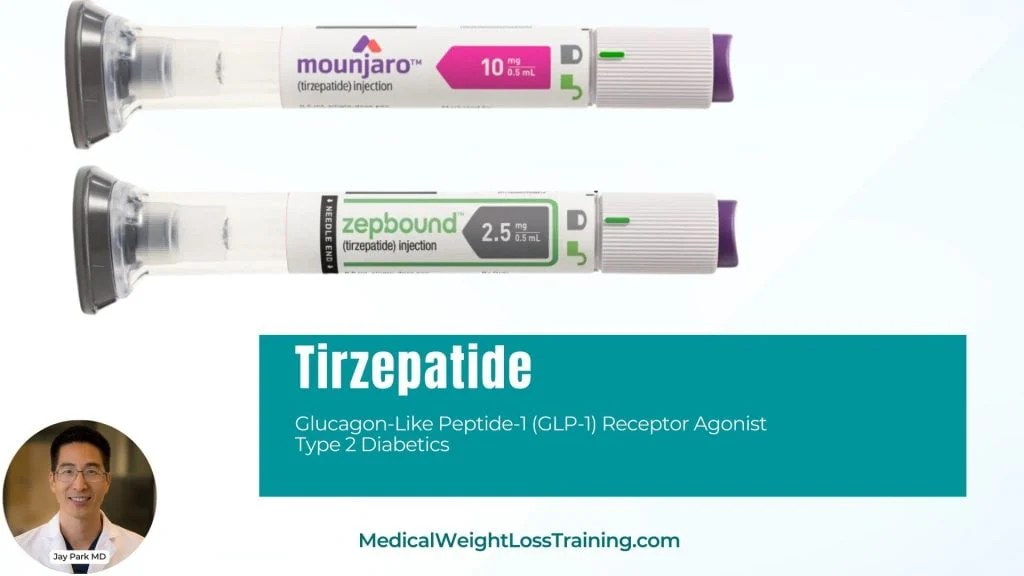
Tirzepatide Release Date For Weight Loss . Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without.
from medicalweightlosstraining.com
For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in.
Tirzepatide Protocol Training as Medical Weight Loss Treatment
Tirzepatide Release Date For Weight Loss Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have.
From dibesity.com
Lilly's Tirzepatide Exceptional Weight Loss Results Dibesity Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes. Tirzepatide Release Date For Weight Loss.
From www.poison.org
Tirzepatide (Zepbound) for Weight Loss Poison Control Tirzepatide Release Date For Weight Loss Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. The plans have been described in an application from nhs england to nice. Tirzepatide Release Date For Weight Loss.
From riomedical.com
Transform Your Life Discover Tirzepatide for Effective Weight Loss Tirzepatide Release Date For Weight Loss The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. If less than 5% of the initial weight has been lost after 6 months on the. Tirzepatide Release Date For Weight Loss.
From www.newyouwellness.net
Tirzepatide for Weight Loss New You Wellness Center Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with. Tirzepatide Release Date For Weight Loss.
From www.msn.com
Tirzepatide gets FDA approval for weight loss Tirzepatide Release Date For Weight Loss For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue. Tirzepatide Release Date For Weight Loss.
From vitalizewellnessnc.com
Tirzepatide Weight Loss Cary, NC Vitalize Wellness Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the. Tirzepatide Release Date For Weight Loss.
From cardiacwire.com
Tirzepatide’s Weight Loss Superiority HeartFlow’s AllInOne Results Tirzepatide Release Date For Weight Loss Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new. Tirzepatide Release Date For Weight Loss.
From www.biochempeg.com
GLP1R Agonists for Weight Loss Biopharma PEG Tirzepatide Release Date For Weight Loss Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Nhs england. Tirzepatide Release Date For Weight Loss.
From www.transformyou.com
Study Shows Tirzepatide Lowers Weight Across All Groups With Obesity Tirzepatide Release Date For Weight Loss Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to. Tirzepatide Release Date For Weight Loss.
From apollovh.com
Exploring the Effectiveness of Tirzepatide for Weight Loss A Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new. Tirzepatide Release Date For Weight Loss.
From www.houstonweightloss.com
Tirzepatide vs Semaglutide for Weight Loss Houston Weight Loss Center Tirzepatide Release Date For Weight Loss For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. Fda approves lilly's zepbound™. Tirzepatide Release Date For Weight Loss.
From muscleandbrawn.com
Tirzepatide Guide Dosages & Sides For Weight Loss Tirzepatide Release Date For Weight Loss Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the. Tirzepatide Release Date For Weight Loss.
From yuniquemedical.com
Tirzepatide as the Next Game Changer in Weight Loss Drugs Yunique Medical Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without.. Tirzepatide Release Date For Weight Loss.
From innopeptides.com
Tirzepatide, a new injectable weight loss medication, is a GIPGLP1 Tirzepatide Release Date For Weight Loss The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment. Tirzepatide Release Date For Weight Loss.
From biopharma.media
Tirzepatide Strong WeightLoss Drug • BioPharma Media Tirzepatide Release Date For Weight Loss The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide. Tirzepatide Release Date For Weight Loss.
From biopharma.media
Tirzepatide Strong WeightLoss Drug • BioPharma Media Tirzepatide Release Date For Weight Loss The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to. Tirzepatide Release Date For Weight Loss.
From biopreventative.com
Tirzepatide Weight Loss Treatment in United States Tirzepatide Release Date For Weight Loss Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. The plans have been described in an application from nhs england to nice that proposes a phased launch of. Tirzepatide Release Date For Weight Loss.
From medicalxpress.com
Tirzepatide weightloss study shows promising results Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. Nhs england has asked nice to consider approving a slow phased rollout. Tirzepatide Release Date For Weight Loss.
From brandonmedicalcenter.com
Exploring Tirzepatide A Revolutionary Approach to Weight Loss Tirzepatide Release Date For Weight Loss Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. The plans have been described in an application from nhs england to nice that proposes a. Tirzepatide Release Date For Weight Loss.
From topdocstore.company.site
Tirzepatide/BPC157 16.6mg/0.5mg/ml 2ml Vial NEW PATIENT STARTER PACK Tirzepatide Release Date For Weight Loss For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking.. Tirzepatide Release Date For Weight Loss.
From www.visualmed.org
SURMOUNT1 Trial Tirzepatide for Weight Loss in Obesity Visualmed Tirzepatide Release Date For Weight Loss Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. If. Tirzepatide Release Date For Weight Loss.
From www.healio.com
Adults with obesity using tirzepatide maintain ‘remarkable’ weight loss Tirzepatide Release Date For Weight Loss Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and. Tirzepatide Release Date For Weight Loss.
From www.outlookindia.com
Tirzepatide Weight Loss (Must Avoid) Side Effects, Dangers And A Safer Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide. Tirzepatide Release Date For Weight Loss.
From medicalweightlosstraining.com
Tirzepatide Protocol Training as Medical Weight Loss Treatment Tirzepatide Release Date For Weight Loss For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. Nhs england. Tirzepatide Release Date For Weight Loss.
From www.ketodirty.com
12 Interesting Tirzepatide Facts You Need To Know Tirzepatide Release Date For Weight Loss The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. Nhs england. Tirzepatide Release Date For Weight Loss.
From facemedstore.com
Tirzepatide Trials Show Potential for Weight Loss and Cardiovascular Tirzepatide Release Date For Weight Loss Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. The plans have been described in an application from nhs england to nice that proposes. Tirzepatide Release Date For Weight Loss.
From www.transformyou.com
Tirzepatide & Weight Loss Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. For many patients on tirzepatide, a transformative medication used for weight loss and. Tirzepatide Release Date For Weight Loss.
From wellnessword.com
Tirzepatide Weight Loss Drug Results Revealed Tirzepatide Release Date For Weight Loss The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. If less than 5% of the initial weight has been lost after 6 months on the. Tirzepatide Release Date For Weight Loss.
From easytrimclinic.com
How Tirzepatide Injections Aid Weight Loss Tirzepatide Release Date For Weight Loss If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. For many patients on tirzepatide, a transformative medication used for weight loss and. Tirzepatide Release Date For Weight Loss.
From thrivecolorado.com
Tirzepatide Weight Loss in Denver Thrive Health, Colorado Tirzepatide Release Date For Weight Loss The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Nhs england. Tirzepatide Release Date For Weight Loss.
From www.vitalityhealthsfl.com
Tirzepatide Therapy Medical Weight Loss Center Vitality Health SFL Tirzepatide Release Date For Weight Loss Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. Fda approves lilly's zepbound™. Tirzepatide Release Date For Weight Loss.
From www.genemedics.com
Tirzepatide for Weight Loss (Fat Loss) Benefits & Side Effects Tirzepatide Release Date For Weight Loss The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without. For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. Tirzepatide, the active ingredient in zepbound and mounjaro, is part of new class of medications used to treat obesity and diabetes that have. If less. Tirzepatide Release Date For Weight Loss.
From www.choosingtherapy.com
Tirzepatide for Weight Loss What You Need to Know Tirzepatide Release Date For Weight Loss Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. The plans have been described in an application from nhs england to nice that proposes a phased launch of. Tirzepatide Release Date For Weight Loss.
From medicalweightlosstraining.com
Tirzepatide Protocol Training as Medical Weight Loss Treatment Tirzepatide Release Date For Weight Loss For many patients on tirzepatide, a transformative medication used for weight loss and diabetes,. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose, decide whether to continue treatment, taking. The plans have been described in an application from nhs england to nice that proposes a phased launch of tirzepatide without.. Tirzepatide Release Date For Weight Loss.
From biopharma.media
Tirzepatide Strong WeightLoss Drug • BioPharma Media Tirzepatide Release Date For Weight Loss Fda approves lilly's zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight. Nhs england has asked nice to consider approving a slow phased rollout of tirzepatide (mounjaro) for weight loss in. If less than 5% of the initial weight has been lost after 6 months on the highest tolerated dose,. Tirzepatide Release Date For Weight Loss.