What Chemical Combusts With Water . A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Why sodium and potassium really explode in water inorganic chemistry: Heat provides the activation energy to start the chemical reaction. The chemical reaction was a simple one: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Many combustion reactions occur with a. Hydrogen combining with oxygen to produce water.
from www.grc.nasa.gov
Many combustion reactions occur with a. Why sodium and potassium really explode in water inorganic chemistry: The chemical reaction was a simple one: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Heat provides the activation energy to start the chemical reaction. Hydrogen combining with oxygen to produce water. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water.
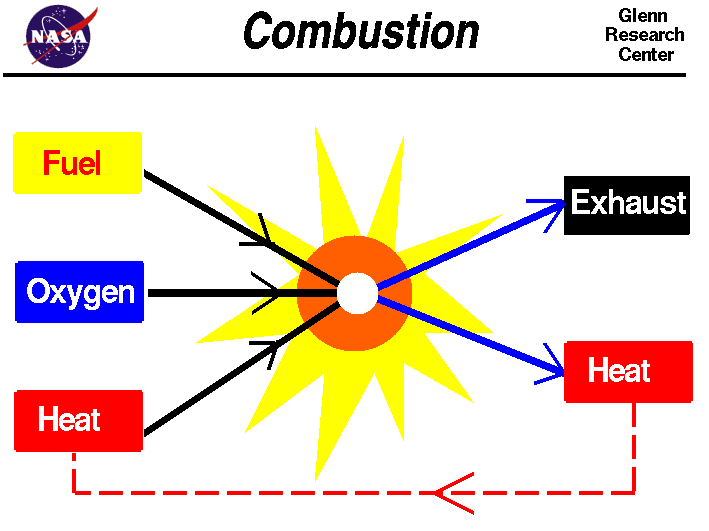
Combustion
What Chemical Combusts With Water The chemical reaction was a simple one: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical reaction. The chemical reaction was a simple one: A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Why sodium and potassium really explode in water inorganic chemistry: Hydrogen combining with oxygen to produce water. Many combustion reactions occur with a.
From www.slideserve.com
PPT Hydrocarbons / Organic Chemistry PowerPoint Presentation, free What Chemical Combusts With Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Many combustion reactions occur with a. The chemical reaction was a simple. What Chemical Combusts With Water.
From www.tessshebaylo.com
Balanced Equation For The Reaction Of Carbon Dioxide And Water What Chemical Combusts With Water The chemical reaction was a simple one: A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Hydrogen combining with oxygen to produce water. Why sodium and potassium really explode in water inorganic chemistry: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and. What Chemical Combusts With Water.
From sciencetallis.weebly.com
7. Organic Chemistry THOMAS TALLIS SCIENCE What Chemical Combusts With Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Why sodium and potassium really explode in water inorganic chemistry: Hydrogen combining. What Chemical Combusts With Water.
From www.numerade.com
SOLVEDLimiting Reactant_Theoretical Yieldand Percent Yield reaction What Chemical Combusts With Water The chemical reaction was a simple one: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Hydrogen combining with oxygen to. What Chemical Combusts With Water.
From slideplayer.com
Types of Chemical Reactions ppt download What Chemical Combusts With Water Why sodium and potassium really explode in water inorganic chemistry: Heat provides the activation energy to start the chemical reaction. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Hydrogen combining with oxygen to produce water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and. What Chemical Combusts With Water.
From www.chegg.com
Solved Ethanol combusts via the following chemical reaction What Chemical Combusts With Water Many combustion reactions occur with a. Hydrogen combining with oxygen to produce water. Heat provides the activation energy to start the chemical reaction. Why sodium and potassium really explode in water inorganic chemistry: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. A combustion reaction, commonly referred to as burning,. What Chemical Combusts With Water.
From tellab.ie
Chemicals in drinking water T.E Laboratories What Chemical Combusts With Water Hydrogen combining with oxygen to produce water. The chemical reaction was a simple one: Why sodium and potassium really explode in water inorganic chemistry: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Many combustion reactions occur with a. A. What Chemical Combusts With Water.
From www.coursehero.com
[Solved] Pentane gas (C5H12) combusts with oxygen gas (O2) to form What Chemical Combusts With Water Why sodium and potassium really explode in water inorganic chemistry: Heat provides the activation energy to start the chemical reaction. The chemical reaction was a simple one: A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Hydrogen combining with oxygen to produce water. Combustion is a reaction. What Chemical Combusts With Water.
From slideplayer.com
Chemical Energy Unit ppt download What Chemical Combusts With Water A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Many combustion reactions occur with a. Why sodium and. What Chemical Combusts With Water.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board What Chemical Combusts With Water The chemical reaction was a simple one: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical reaction. Many combustion reactions occur with a. Hydrogen combining with oxygen to produce water. All. What Chemical Combusts With Water.
From www.coursehero.com
[Solved] Pentane gas (C5H12) combusts with oxygen gas (O2) to form What Chemical Combusts With Water A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Hydrogen combining with oxygen to produce water. Many combustion reactions occur with a. Heat provides the activation energy to start the chemical reaction. The chemical reaction was a simple one: All of group 1 elements —lithium, sodium, potassium,. What Chemical Combusts With Water.
From studylib.net
COMBUSTION of PROPANE What Chemical Combusts With Water Why sodium and potassium really explode in water inorganic chemistry: The chemical reaction was a simple one: Heat provides the activation energy to start the chemical reaction. Many combustion reactions occur with a. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Hydrogen combining with oxygen to. What Chemical Combusts With Water.
From www.youtube.com
Balancing Combustion Reactions YouTube What Chemical Combusts With Water Hydrogen combining with oxygen to produce water. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. The chemical reaction was a simple one: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water. What Chemical Combusts With Water.
From www.youtube.com
Balanced Equation for the Combustion of Methane (CH4) YouTube What Chemical Combusts With Water The chemical reaction was a simple one: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood,. What Chemical Combusts With Water.
From www.worksheetsplanet.com
Chemical Properties of Water What Chemical Combusts With Water Why sodium and potassium really explode in water inorganic chemistry: Hydrogen combining with oxygen to produce water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Many combustion reactions occur with a. The chemical reaction was a simple one: A combustion reaction, commonly referred to as burning, usually occurs when. What Chemical Combusts With Water.
From dokumen.tips
(PPT) Complete the following reactions Lithium Sulfate dissolves in What Chemical Combusts With Water Heat provides the activation energy to start the chemical reaction. Why sodium and potassium really explode in water inorganic chemistry: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Many combustion reactions occur with a. Hydrogen combining with oxygen to produce water. Combustion is a reaction between a hydrocarbon fuel. What Chemical Combusts With Water.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID3551913 What Chemical Combusts With Water Hydrogen combining with oxygen to produce water. The chemical reaction was a simple one: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Heat provides the activation energy to start the chemical reaction. Many combustion reactions occur with a. A combustion reaction, commonly referred to as burning, usually occurs when. What Chemical Combusts With Water.
From ar.inspiredpencil.com
Gasoline Combustion Reaction What Chemical Combusts With Water A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Heat provides the activation energy to start the chemical reaction. Hydrogen combining with oxygen to produce water. The chemical reaction was a simple one: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and. What Chemical Combusts With Water.
From www.youtube.com
Combustion of Hydrocarbon Carbon Compound YouTube What Chemical Combusts With Water Hydrogen combining with oxygen to produce water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Many combustion reactions occur with a. Heat provides the activation energy to start the chemical reaction. Why sodium and potassium really explode in water inorganic chemistry: Combustion is a reaction between a hydrocarbon fuel. What Chemical Combusts With Water.
From www.numerade.com
SOLVED Isopropanol (C3H8O) combusts with oxygen gas (O2) to form What Chemical Combusts With Water Why sodium and potassium really explode in water inorganic chemistry: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. The chemical reaction was a simple one: Heat provides. What Chemical Combusts With Water.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples What Chemical Combusts With Water The chemical reaction was a simple one: A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Heat provides the activation energy to start the chemical reaction. Why sodium and potassium really explode in water inorganic chemistry: Many combustion reactions occur with a. All of group 1 elements. What Chemical Combusts With Water.
From www.youtube.com
combustion of propane YouTube What Chemical Combusts With Water Many combustion reactions occur with a. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Why sodium and potassium really explode in water inorganic chemistry: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co. What Chemical Combusts With Water.
From www.worldatlas.com
What Are The Properties Of Matter? WorldAtlas What Chemical Combusts With Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Hydrogen combining with oxygen to produce water. The chemical reaction was a simple one: Many combustion reactions occur with a. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and. What Chemical Combusts With Water.
From www.youtube.com
How to Balance C6H6 + O2 = CO2 + H2O of Benzene) YouTube What Chemical Combusts With Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Many combustion reactions occur with a. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to. What Chemical Combusts With Water.
From www.coursehero.com
[Solved] 3. Pentane combusts with oxygen to form carbon dioxide and What Chemical Combusts With Water Why sodium and potassium really explode in water inorganic chemistry: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical reaction. The chemical reaction was a simple one: A combustion reaction, commonly. What Chemical Combusts With Water.
From www.chegg.com
4. When ignited, ethanol combusts in oxygen and What Chemical Combusts With Water Hydrogen combining with oxygen to produce water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Many combustion reactions occur with a. Heat provides the activation energy to start the chemical reaction. A combustion reaction, commonly referred to as burning,. What Chemical Combusts With Water.
From www.slideserve.com
PPT Reactions of Hydrocarbons Combustion PowerPoint Presentation What Chemical Combusts With Water Hydrogen combining with oxygen to produce water. Heat provides the activation energy to start the chemical reaction. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Why sodium and potassium really explode in water inorganic chemistry: The chemical reaction was a simple one: Many combustion reactions occur with a. Combustion. What Chemical Combusts With Water.
From www.grc.nasa.gov
Combustion What Chemical Combusts With Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. The chemical reaction was a simple one: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy. What Chemical Combusts With Water.
From slideplayer.com
Physical or Chemical Change? Vocabulary Chemistry. ppt download What Chemical Combusts With Water A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. The chemical reaction was a simple one: Many combustion reactions occur with a. Why sodium and potassium really explode in water inorganic chemistry: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. What Chemical Combusts With Water.
From addielangchem.blogspot.com
PreAP Chem 2015 What Chemical Combusts With Water The chemical reaction was a simple one: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Many combustion reactions occur with a. Combustion is a reaction between a. What Chemical Combusts With Water.
From www.chegg.com
Solved When propane (C3H8) reacts with oxygen, carbon What Chemical Combusts With Water The chemical reaction was a simple one: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical reaction. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or. What Chemical Combusts With Water.
From slideplayer.com
Changes in Matter Chapter 2 Section 2 ppt download What Chemical Combusts With Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Why sodium and potassium really explode in water inorganic chemistry: The chemical. What Chemical Combusts With Water.
From sklep.foteks.pl
Plakat Combustion Reaction Infographic Diagram with example of What Chemical Combusts With Water Why sodium and potassium really explode in water inorganic chemistry: The chemical reaction was a simple one: Hydrogen combining with oxygen to produce water. Many combustion reactions occur with a. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane,. What Chemical Combusts With Water.
From www.chegg.com
Solved Methane combusts according to the following chemical What Chemical Combusts With Water A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Why sodium and potassium really explode in water inorganic chemistry: Heat provides the activation energy to start the chemical reaction. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. What Chemical Combusts With Water.
From www.youtube.com
Gas Stoichiometry Combustion of acetylene YouTube What Chemical Combusts With Water Many combustion reactions occur with a. Heat provides the activation energy to start the chemical reaction. Why sodium and potassium really explode in water inorganic chemistry: Hydrogen combining with oxygen to produce water. A combustion reaction, commonly referred to as burning, usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. The chemical reaction was a. What Chemical Combusts With Water.